MENU
The Electronic Scholarly Publishing Project: Providing world-wide, free access to classic scientific papers and other scholarly materials, since 1993.
More About: ESP | OUR CONTENT | THIS WEBSITE | WHAT'S NEW | WHAT'S HOT
ESP: PubMed Auto Bibliography 21 Feb 2026 at 01:30 Created:
Horizontal Gene Transfer
The pathology-inducing genes of O157:H7 appear to have been acquired, likely via prophage, by a nonpathogenic E. coli ancestor, perhaps 20,000 years ago. That is, horizontal gene transfer (HGT) can lead to the profound phenotypic change from benign commensal to lethal pathogen. "Horizontal" in this context refers to the lateral or "sideways" movement of genes between microbes via mechanisms not directly associated with reproduction. HGT among prokaryotes can occur between members of the same "species" as well as between microbes separated by vast taxonomic distances. As such, much prokaryotic genetic diversity is both created and sustained by high levels of HGT. Although HGT can occur for genes in the core-genome component of a pan-genome, it occurs much more frequently among genes in the optional, flex-genome component. In some cases, HGT has become so common that it is possible to think of some "floating" genes more as attributes of the environment in which they are useful rather than as attributes of any individual bacterium or strain or "species" that happens to carry them. For example, bacterial plasmids that occur in hospitals are capable of conferring pathogenicity on any bacterium that successfully takes them up. This kind of genetic exchange can occur between widely unrelated taxa.
Created with PubMed® Query: ( "horizontal gene transfer" OR "lateral gene transfer") NOT pmcbook NOT ispreviousversion
Citations The Papers (from PubMed®)
RevDate: 2026-02-20
Comparative Genomic and Transcriptomic Analysis Reveals Why Paenarthrobacter Strains Are Specialists in the Degradation of the Fungicide Iprodione.
Microbial biotechnology, 19(2):e70319.
Paenarthrobacters degrade the fungicide iprodione through a pathway involving an amidase (IpaH), a deacetylase (DdaH) and a hydrolase (DuaH). We aimed to elucidate the mechanisms of this catabolic specialisation and its evolution in Paenarthrobacters. Two new iprodione-degrading Paenarthrobacter strains TA1.8 and C1 were sequenced, and their genomes were analysed comparatively to the iprodione-degrading Paenarthrobacter strains YJN-5 and YJN-D. We noted different gene organisation motifs amongst strains, suggesting different stages of pathway evolution in the studied strains depending on their prior exposure to iprodione. Strains derived from soils exposed to iprodione (TA1.8, YJN-5 and YJN-D) carry multiple copies of ipaH, ddaH and duaH. Conversely, strain C1, isolated from a pristine soil, carried one copy of the set. Comparative genomics and pangenome analysis of Paenarthrobacters suggested an evolution route of the iprodione transformation pathway which involves acquisition of ddaH through horizontal gene transfer, gene duplication of the chromosomally encoded ipaH and ddaH, and further genetic rearrangements for pathway optimisation, complementing duaH, a core gene in Paenarthrobacters. Transcriptomic analysis of TA1.8 verified the importance of all ipaH, ddaH and duaH homologues in iprodione transformation and pointed to hydantoinases as potential facilitators of the transformation of the hydantoin-containing intermediate N-(3,-5-dichlorophenyl)-2,4-dioxoimida-zolidine, a step mediated by DdaH.
Additional Links: PMID-41721206
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41721206,
year = {2026},
author = {Michelioudakis, V and Zafranas, A and Myrisiotis, C and Makri, S and Katsoula, A and Campos, M and Vasileiadis, S and Karpouzas, DG},
title = {Comparative Genomic and Transcriptomic Analysis Reveals Why Paenarthrobacter Strains Are Specialists in the Degradation of the Fungicide Iprodione.},
journal = {Microbial biotechnology},
volume = {19},
number = {2},
pages = {e70319},
doi = {10.1111/1751-7915.70319},
pmid = {41721206},
issn = {1751-7915},
support = {MIS 5002636//Omic-Engine RI project/ ; //Co-financed Greece and European Union/ ; //European Regional Development Fund/ ; },
abstract = {Paenarthrobacters degrade the fungicide iprodione through a pathway involving an amidase (IpaH), a deacetylase (DdaH) and a hydrolase (DuaH). We aimed to elucidate the mechanisms of this catabolic specialisation and its evolution in Paenarthrobacters. Two new iprodione-degrading Paenarthrobacter strains TA1.8 and C1 were sequenced, and their genomes were analysed comparatively to the iprodione-degrading Paenarthrobacter strains YJN-5 and YJN-D. We noted different gene organisation motifs amongst strains, suggesting different stages of pathway evolution in the studied strains depending on their prior exposure to iprodione. Strains derived from soils exposed to iprodione (TA1.8, YJN-5 and YJN-D) carry multiple copies of ipaH, ddaH and duaH. Conversely, strain C1, isolated from a pristine soil, carried one copy of the set. Comparative genomics and pangenome analysis of Paenarthrobacters suggested an evolution route of the iprodione transformation pathway which involves acquisition of ddaH through horizontal gene transfer, gene duplication of the chromosomally encoded ipaH and ddaH, and further genetic rearrangements for pathway optimisation, complementing duaH, a core gene in Paenarthrobacters. Transcriptomic analysis of TA1.8 verified the importance of all ipaH, ddaH and duaH homologues in iprodione transformation and pointed to hydantoinases as potential facilitators of the transformation of the hydantoin-containing intermediate N-(3,-5-dichlorophenyl)-2,4-dioxoimida-zolidine, a step mediated by DdaH.},
}
RevDate: 2026-02-20
Antimicrobial resistance gene diversity, prevalence, and mobility within four landfills.
Canadian journal of microbiology [Epub ahead of print].
Antibiotics in landfills create selection pressures on the microorganisms present, selecting for antibiotic resistance genes (ARGs) and antibiotic resistant organisms (ARO). The aim of this study was to assess whether landfills are hot-spots of antimicrobial resistance and whether landfills may contribute to global ARO diversity through ARG lateral gene transfer. Genome resolved metagenomic sequencing combined with sequence-search-based and deep learning tools were used to determine ARG diversity and prevalence from four active municipal landfills and their adjacent ground or surface water systems. Comparison to pristine and anthropogenic environments highlighted that landfill microbial communities contain distinct ARG signatures, including a broader diversity of ARGs. Plasmids made up 4.1-8.4% of assembled scaffolds and carried 5.4-12.0% of the identified ARGs in assembled data, depending on the sample type. Enriched ARG resistance mechanisms on mobile elements included multidrug resistance and antibiotic inactivation. The results indicate that landfills house a high diversity of antimicrobial resistance mechanisms and drug classes, with a moderate fraction encoded on mobile elements. Landfills are thus likely mixing grounds for ARG transfer and evolution of novel or augmented ARO lineages.
Additional Links: PMID-41719533
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41719533,
year = {2026},
author = {Ippolito, I and Hug, L},
title = {Antimicrobial resistance gene diversity, prevalence, and mobility within four landfills.},
journal = {Canadian journal of microbiology},
volume = {},
number = {},
pages = {},
doi = {10.1139/cjm-2025-0226},
pmid = {41719533},
issn = {1480-3275},
abstract = {Antibiotics in landfills create selection pressures on the microorganisms present, selecting for antibiotic resistance genes (ARGs) and antibiotic resistant organisms (ARO). The aim of this study was to assess whether landfills are hot-spots of antimicrobial resistance and whether landfills may contribute to global ARO diversity through ARG lateral gene transfer. Genome resolved metagenomic sequencing combined with sequence-search-based and deep learning tools were used to determine ARG diversity and prevalence from four active municipal landfills and their adjacent ground or surface water systems. Comparison to pristine and anthropogenic environments highlighted that landfill microbial communities contain distinct ARG signatures, including a broader diversity of ARGs. Plasmids made up 4.1-8.4% of assembled scaffolds and carried 5.4-12.0% of the identified ARGs in assembled data, depending on the sample type. Enriched ARG resistance mechanisms on mobile elements included multidrug resistance and antibiotic inactivation. The results indicate that landfills house a high diversity of antimicrobial resistance mechanisms and drug classes, with a moderate fraction encoded on mobile elements. Landfills are thus likely mixing grounds for ARG transfer and evolution of novel or augmented ARO lineages.},
}
RevDate: 2026-02-20
CmpDate: 2026-02-20
Convergent gut microbiome adaptation and pervasive antibiotic resistome in Qinghai-Tibet Plateau passerines.
Frontiers in microbiology, 16:1733974.
INTRODUCTION: The Qinghai-Tibet Plateau, an extreme high-altitude ecosystem, presents a unique model for studying host-microbe-environment coevolution under environmental stress. However, the role of resident wildlife, particularly non-migratory passerines, as reservoirs and vectors for cross-boundary antibiotic resistance gene (ARG) dissemination remains poorly understood.
METHODS: Here, through metagenomic analysis of two endemic passerines (Pseudopodoces humilis and Pyrgilauda ruficollis) and their habitats.
RESULTS: We reveal convergent adaptations in their gut microbiomes, dominated by Actinomycetota, Pseudomonadota and Bacillota. Functional enrichment in carbohydrate metabolism and genetic information processing underpins host energy optimization in extreme high-altitude environments. Critically, these birds constitute a major reservoir of ARGs, harboring 153 antibiotic resistance ontologies (AROs) with nearly universal resistance to clinical antibiotic classes. The core resistome-comprising glycopeptide (van clusters), fluoroquinolone, and tetracycline resistance genes-reflects anthropogenic contamination amplified by environmental persistence. Environmental transmission pathways were unequivocally demonstrated via 47 AROs shared between avian hosts and proximal matrices (soil/grass), coupled with livestock-derived antibiotic influx through excreta, establishing the plateau as a hotspot for resistance gene flux. Strikingly, "low-abundance-high-resistance" taxa (Pseudomonadota, Actinomycetota, and Bacillota; ≤30% abundance but >80% ARG contribution) drive resistome plasticity, potentially facilitated by horizontal gene transfer.
DISCUSSION: Our findings redefine resident passerines as sentinels of ecosystem health and bridges for cross-boundary antimicrobial resistance (AMR) spread. Mitigating global AMR thus necessitates interdisciplinary strategies targeting environmental reservoirs (e.g., regulating livestock antibiotic use) and monitoring avian-mediated gene flow.
Additional Links: PMID-41717089
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41717089,
year = {2025},
author = {Shi, S and Qi, J and Peng, W and Su, X and Chen, P and Xu, S and Li, S and Ma, L and Wang, W and Jiang, K and Liu, Z and Li, W and Xiong, H and Wang, Y},
title = {Convergent gut microbiome adaptation and pervasive antibiotic resistome in Qinghai-Tibet Plateau passerines.},
journal = {Frontiers in microbiology},
volume = {16},
number = {},
pages = {1733974},
pmid = {41717089},
issn = {1664-302X},
abstract = {INTRODUCTION: The Qinghai-Tibet Plateau, an extreme high-altitude ecosystem, presents a unique model for studying host-microbe-environment coevolution under environmental stress. However, the role of resident wildlife, particularly non-migratory passerines, as reservoirs and vectors for cross-boundary antibiotic resistance gene (ARG) dissemination remains poorly understood.
METHODS: Here, through metagenomic analysis of two endemic passerines (Pseudopodoces humilis and Pyrgilauda ruficollis) and their habitats.
RESULTS: We reveal convergent adaptations in their gut microbiomes, dominated by Actinomycetota, Pseudomonadota and Bacillota. Functional enrichment in carbohydrate metabolism and genetic information processing underpins host energy optimization in extreme high-altitude environments. Critically, these birds constitute a major reservoir of ARGs, harboring 153 antibiotic resistance ontologies (AROs) with nearly universal resistance to clinical antibiotic classes. The core resistome-comprising glycopeptide (van clusters), fluoroquinolone, and tetracycline resistance genes-reflects anthropogenic contamination amplified by environmental persistence. Environmental transmission pathways were unequivocally demonstrated via 47 AROs shared between avian hosts and proximal matrices (soil/grass), coupled with livestock-derived antibiotic influx through excreta, establishing the plateau as a hotspot for resistance gene flux. Strikingly, "low-abundance-high-resistance" taxa (Pseudomonadota, Actinomycetota, and Bacillota; ≤30% abundance but >80% ARG contribution) drive resistome plasticity, potentially facilitated by horizontal gene transfer.
DISCUSSION: Our findings redefine resident passerines as sentinels of ecosystem health and bridges for cross-boundary antimicrobial resistance (AMR) spread. Mitigating global AMR thus necessitates interdisciplinary strategies targeting environmental reservoirs (e.g., regulating livestock antibiotic use) and monitoring avian-mediated gene flow.},
}
RevDate: 2026-02-20
Cross-kingdom genomic variation in chicken gut microbiomes: insights from China's diverse local breeds.
Microbiome pii:10.1186/s40168-026-02347-3 [Epub ahead of print].
BACKGROUND: The gut microbiome possesses substantial genetic diversity that supports microbial adaptation, but the genomic variation patterns across its prokaryotic and viral populations remain incompletely characterized.
RESULTS: Through integrated metagenomic and metatranscriptomic analysis of ten indigenous chicken breeds from China, we recovered 1527 representative prokaryotic MAGs, 37,555 representative DNA viral contigs, and 1867 representative RNA viral contigs (primarily comprising Bacillota/Bacteroidota, Uroviricota, and Lenarviricota/Pisuviricota, respectively). By integrating complementary short-read and long-read metagenomics with metatranscriptomics, we identified structural variants (SVs) and single-nucleotide variants (SNVs) in these cross-kingdom genomes. Positive SV-SNV density correlations occurred consistently across all microbial groups, indicating coordinated mutational processes. DNA viruses exhibited the highest variant prevalence (86.9% SNVs, 47.7% SVs), with temperate phages accumulating significantly more variants than virulent phages. Functionally, prokaryotic variants accumulated in carbohydrate metabolism and amino acid metabolism, while viral variants demonstrated broad metabolic hijacking. Horizontal gene transfer (HGT) was characterized by a strong virus-associated signature (69.40% of 536 events) and marked by an asymmetric pattern, with phage-to-bacteria (P-to-B) flow alone constituting 37.50% of all events. Random forest analysis revealed a strong bidirectional predictive relationship between SV and SNV densities across prokaryotic, DNA viral, and RNA viral populations, suggesting coupled genomic instability. Niche breadth emerged as a major driver of SNVs across kingdoms and was positively correlated with variant density. In prokaryotes, HGT events significantly shaped variant patterns. For viruses, genomic GC content was an important factor and consistently showed a negative correlation with SNV density in both DNA and RNA viruses.
CONCLUSIONS: These findings demonstrate that coordinated mutational processes and kingdom-specific intrinsic factors drive genomic variation, with viruses serving as key genetic exchange vectors in chicken gut ecosystems. Video Abstract.
Additional Links: PMID-41715166
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41715166,
year = {2026},
author = {Zhang, J and Xu, L and Ge, X and Zi, X and Chen, S and Liu, C and Wang, K and Zhou, J and Dou, T and Wong, JWC and Lin, Q and Kang, X and Cao, Z},
title = {Cross-kingdom genomic variation in chicken gut microbiomes: insights from China's diverse local breeds.},
journal = {Microbiome},
volume = {},
number = {},
pages = {},
doi = {10.1186/s40168-026-02347-3},
pmid = {41715166},
issn = {2049-2618},
support = {2024A1515140076//Guangdong Basic and Applied Basic Research Foundation/ ; 202401AU070079//Yunnan Fundamental Research Projects/ ; 221110133//Dongguan University of Technology Top Talent Professor Start Up Fund/ ; 202301BD070001-136//Key Project of Yunnan Province Agricultural Joint Special Project/ ; 202305AC160040//Yunnan Province Young and Middle-aged Academic and Technical Leader Reserve Talent Project/ ; },
abstract = {BACKGROUND: The gut microbiome possesses substantial genetic diversity that supports microbial adaptation, but the genomic variation patterns across its prokaryotic and viral populations remain incompletely characterized.
RESULTS: Through integrated metagenomic and metatranscriptomic analysis of ten indigenous chicken breeds from China, we recovered 1527 representative prokaryotic MAGs, 37,555 representative DNA viral contigs, and 1867 representative RNA viral contigs (primarily comprising Bacillota/Bacteroidota, Uroviricota, and Lenarviricota/Pisuviricota, respectively). By integrating complementary short-read and long-read metagenomics with metatranscriptomics, we identified structural variants (SVs) and single-nucleotide variants (SNVs) in these cross-kingdom genomes. Positive SV-SNV density correlations occurred consistently across all microbial groups, indicating coordinated mutational processes. DNA viruses exhibited the highest variant prevalence (86.9% SNVs, 47.7% SVs), with temperate phages accumulating significantly more variants than virulent phages. Functionally, prokaryotic variants accumulated in carbohydrate metabolism and amino acid metabolism, while viral variants demonstrated broad metabolic hijacking. Horizontal gene transfer (HGT) was characterized by a strong virus-associated signature (69.40% of 536 events) and marked by an asymmetric pattern, with phage-to-bacteria (P-to-B) flow alone constituting 37.50% of all events. Random forest analysis revealed a strong bidirectional predictive relationship between SV and SNV densities across prokaryotic, DNA viral, and RNA viral populations, suggesting coupled genomic instability. Niche breadth emerged as a major driver of SNVs across kingdoms and was positively correlated with variant density. In prokaryotes, HGT events significantly shaped variant patterns. For viruses, genomic GC content was an important factor and consistently showed a negative correlation with SNV density in both DNA and RNA viruses.
CONCLUSIONS: These findings demonstrate that coordinated mutational processes and kingdom-specific intrinsic factors drive genomic variation, with viruses serving as key genetic exchange vectors in chicken gut ecosystems. Video Abstract.},
}
RevDate: 2026-02-19
Phylogenomic and population genomic insights into the dissemination of ESBL-producing Escherichia coli causing bloodstream infections in the United Arab Emirates.
Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases pii:S1567-1348(26)00029-8 [Epub ahead of print].
Extended-spectrum β-lactamase-producing Escherichia coli are globally disseminated pathogens whose success is driven by clonal expansion and horizontal gene transfer. However, the population structure and evolutionary relationships of these organisms in the United Arab Emirates remain insufficiently characterized. In this study, we applied a population genomic and phylogenomic approach to investigate ESBL-producing E. coli causing bloodstream infections and their genetic relatedness to strains from non-human reservoirs within a One Health framework. Forty-five ESBL-producing E. coli isolates recovered from bloodstream infections between 2021 and 2024 were analyzed, with whole-genome sequencing performed on 29 representative isolates. Genomic analyses revealed the predominance of internationally disseminated high-risk lineages, particularly sequence types ST131 and ST1193, largely associated with the ESBL gene blaCTX-M-15. Conserved genetic contexts of blaCTX-M-15 in these lineages suggested stable vertical inheritance, whereas greater diversity of mobile genetic elements was observed among non-ST131 isolates, indicating ongoing horizontal gene transfer. Additional resistance determinants, including blaDHA-1, blaSHV-12, and notably the carbapenemase gene blaNDM-5, contributed to multidrug-resistant genotypes, indicating the coexistence of ESBL and carbapenemase activity in a subset of isolates. Phylogenomic comparisons based on core genome variation demonstrated close genetic relatedness between clinical isolates and E. coli from food, poultry, and environmental sources in the United Arab Emirates. These findings indicate that bloodstream infections are associated with shared circulating ESBL-producing E. coli lineages exhibiting genetic relatedness across human and non-human reservoirs. The results highlight the evolutionary connectivity of E. coli populations and emphasize the importance of integrated genomic surveillance to track and limit the spread of multidrug-resistant pathogens.
Additional Links: PMID-41713669
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41713669,
year = {2026},
author = {Khalifa, HO and Mohammed, T and Ramadan, H and Abdalla, A and Ghazawi, A and Al-Marzooq, F},
title = {Phylogenomic and population genomic insights into the dissemination of ESBL-producing Escherichia coli causing bloodstream infections in the United Arab Emirates.},
journal = {Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases},
volume = {},
number = {},
pages = {105905},
doi = {10.1016/j.meegid.2026.105905},
pmid = {41713669},
issn = {1567-7257},
abstract = {Extended-spectrum β-lactamase-producing Escherichia coli are globally disseminated pathogens whose success is driven by clonal expansion and horizontal gene transfer. However, the population structure and evolutionary relationships of these organisms in the United Arab Emirates remain insufficiently characterized. In this study, we applied a population genomic and phylogenomic approach to investigate ESBL-producing E. coli causing bloodstream infections and their genetic relatedness to strains from non-human reservoirs within a One Health framework. Forty-five ESBL-producing E. coli isolates recovered from bloodstream infections between 2021 and 2024 were analyzed, with whole-genome sequencing performed on 29 representative isolates. Genomic analyses revealed the predominance of internationally disseminated high-risk lineages, particularly sequence types ST131 and ST1193, largely associated with the ESBL gene blaCTX-M-15. Conserved genetic contexts of blaCTX-M-15 in these lineages suggested stable vertical inheritance, whereas greater diversity of mobile genetic elements was observed among non-ST131 isolates, indicating ongoing horizontal gene transfer. Additional resistance determinants, including blaDHA-1, blaSHV-12, and notably the carbapenemase gene blaNDM-5, contributed to multidrug-resistant genotypes, indicating the coexistence of ESBL and carbapenemase activity in a subset of isolates. Phylogenomic comparisons based on core genome variation demonstrated close genetic relatedness between clinical isolates and E. coli from food, poultry, and environmental sources in the United Arab Emirates. These findings indicate that bloodstream infections are associated with shared circulating ESBL-producing E. coli lineages exhibiting genetic relatedness across human and non-human reservoirs. The results highlight the evolutionary connectivity of E. coli populations and emphasize the importance of integrated genomic surveillance to track and limit the spread of multidrug-resistant pathogens.},
}
RevDate: 2026-02-19
Evolutionary mobility and genetic dynamics of MORFFO genes: shuttling among ancient plant lineages.
The New phytologist [Epub ahead of print].
Plastid genomes (plastomes) of land plants are characterized by their architectural and genic content stability. However, fern plastomes exhibit unexpected dynamism, characterized by the presence of mobile protein-coding genes (CDS) - Mobile Open Reading Frames in Fern Organelles (MORFFOs). We investigate the evolutionary dynamics of MORFFOs in 30 species of Anemiaceae (Schizaeales), an ancient lineage of ferns, focusing on their transposition, substitution patterns, codon usages, and RNA editing patterns. MORFFOs expand plastome size and occur in diverse intergenic regions, exhibiting dynamic locations, genealogies, and exceptionally high substitution rates compared with canonical plastid CDS. Sliding window and codon usage analyses demonstrate that MORFFOs are under purifying selection but exhibit distinct codon preferences that deviate from those of other plastid CDS, suggesting functional constraints. Phylogenetic incongruence between MORFFOs and other plastid CDS, along with their extraordinary substitution rates and mobility, implies their replication outside plastids. Our findings highlight that MORFFOs are dynamic, potentially selfish genetic elements capable of transcription, translation, and replication independently from plastomes, and fern plastomes might acquire these mobile CDS through frequent horizontal gene transfer and possibly intracellular gene transfer.
Additional Links: PMID-41714151
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41714151,
year = {2026},
author = {Labiak, PH and Kuo, LY and Fauskee, BD and Karol, KG},
title = {Evolutionary mobility and genetic dynamics of MORFFO genes: shuttling among ancient plant lineages.},
journal = {The New phytologist},
volume = {},
number = {},
pages = {},
doi = {10.1111/nph.70986},
pmid = {41714151},
issn = {1469-8137},
support = {303330/2022-8//Conselho Nacional de Desenvolvimento Científico e Tecnológico/ ; NSTC 114-2621-B-007-001//National Science and Technology Council in Taiwan/ ; //Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/ ; },
abstract = {Plastid genomes (plastomes) of land plants are characterized by their architectural and genic content stability. However, fern plastomes exhibit unexpected dynamism, characterized by the presence of mobile protein-coding genes (CDS) - Mobile Open Reading Frames in Fern Organelles (MORFFOs). We investigate the evolutionary dynamics of MORFFOs in 30 species of Anemiaceae (Schizaeales), an ancient lineage of ferns, focusing on their transposition, substitution patterns, codon usages, and RNA editing patterns. MORFFOs expand plastome size and occur in diverse intergenic regions, exhibiting dynamic locations, genealogies, and exceptionally high substitution rates compared with canonical plastid CDS. Sliding window and codon usage analyses demonstrate that MORFFOs are under purifying selection but exhibit distinct codon preferences that deviate from those of other plastid CDS, suggesting functional constraints. Phylogenetic incongruence between MORFFOs and other plastid CDS, along with their extraordinary substitution rates and mobility, implies their replication outside plastids. Our findings highlight that MORFFOs are dynamic, potentially selfish genetic elements capable of transcription, translation, and replication independently from plastomes, and fern plastomes might acquire these mobile CDS through frequent horizontal gene transfer and possibly intracellular gene transfer.},
}
RevDate: 2026-02-19
Microcystins 'steer' antibiotic resistome dynamics by synergetic metabolism and horizontal gene transfer in a megacity's water supply catchment microbiota.
Journal of hazardous materials, 505:141525 pii:S0304-3894(26)00503-0 [Epub ahead of print].
The proliferation of Microcystis has been linked to the widespread occurrence of antibiotic resistance genes (ARGs). Yet, the underlying mechanisms driven by the proliferation-induced microbial metabolic interactions and elevated microcystins (MCs) levels remain unclear. Here, through a year-long field study conducted in Shanghai's largest drinking water supply catchment, we demonstrated that Microcystis proliferation significantly increased ARG relative abundance (by 0.28 ± 0.05 log10(RPKM+1), corresponding to an approximately 60 % increase in abundance; P < 0.05, n = 63) and markedly reshaped the resistome structure (PERMANOVA, P < 0.01). During the whole Microcystis biomass cycle, the MCs were identified as the most predominant driver of the dynamics of waterborne ARGs (SNPs-RDA > 0.6, P < 0.01). Metagenomic binning and metabolic network reconstruction revealed that MC enhanced metabolic cooperation between ARG hosts and surrounding microorganisms (iNAP, Student's T-test, P < 0.001), suggesting MC-involved and nutrient co-metabolism that facilitated persistence of ARGs and the associated bacteria. Furthermore, plasmid conjugation experiments indicated that MCs significantly elevated plasmid-mediated ARG-transfer efficiency by twofold (Wilcoxon test, P < 0.05), promoting the spread of multidrug-resistant genes such as MexB, which may enable MCs to efflux. To quantify these effects, an MC index (MI) and a physiochemical index (PI) were developed, co-explaining > 80 % of ARG variation and identifying dissemination thresholds (TITAN, MI > 0.490 and PI > -0.032) for dominant resistance types. Our findings highlight MC as a natural promoter of ARG transmission, and the proposed indices offer viable tools for monitoring and mitigating antibiotic resistance in drinking water sources.
Additional Links: PMID-41713270
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41713270,
year = {2026},
author = {Jiao, X and Ji, W and Zhang, X and Zhang, S and Dolfing, J and Yang, K and Xie, B and Zhang, Y and Feng, J and Wu, D},
title = {Microcystins 'steer' antibiotic resistome dynamics by synergetic metabolism and horizontal gene transfer in a megacity's water supply catchment microbiota.},
journal = {Journal of hazardous materials},
volume = {505},
number = {},
pages = {141525},
doi = {10.1016/j.jhazmat.2026.141525},
pmid = {41713270},
issn = {1873-3336},
abstract = {The proliferation of Microcystis has been linked to the widespread occurrence of antibiotic resistance genes (ARGs). Yet, the underlying mechanisms driven by the proliferation-induced microbial metabolic interactions and elevated microcystins (MCs) levels remain unclear. Here, through a year-long field study conducted in Shanghai's largest drinking water supply catchment, we demonstrated that Microcystis proliferation significantly increased ARG relative abundance (by 0.28 ± 0.05 log10(RPKM+1), corresponding to an approximately 60 % increase in abundance; P < 0.05, n = 63) and markedly reshaped the resistome structure (PERMANOVA, P < 0.01). During the whole Microcystis biomass cycle, the MCs were identified as the most predominant driver of the dynamics of waterborne ARGs (SNPs-RDA > 0.6, P < 0.01). Metagenomic binning and metabolic network reconstruction revealed that MC enhanced metabolic cooperation between ARG hosts and surrounding microorganisms (iNAP, Student's T-test, P < 0.001), suggesting MC-involved and nutrient co-metabolism that facilitated persistence of ARGs and the associated bacteria. Furthermore, plasmid conjugation experiments indicated that MCs significantly elevated plasmid-mediated ARG-transfer efficiency by twofold (Wilcoxon test, P < 0.05), promoting the spread of multidrug-resistant genes such as MexB, which may enable MCs to efflux. To quantify these effects, an MC index (MI) and a physiochemical index (PI) were developed, co-explaining > 80 % of ARG variation and identifying dissemination thresholds (TITAN, MI > 0.490 and PI > -0.032) for dominant resistance types. Our findings highlight MC as a natural promoter of ARG transmission, and the proposed indices offer viable tools for monitoring and mitigating antibiotic resistance in drinking water sources.},
}
RevDate: 2026-02-19
CmpDate: 2026-02-19
A One Health study of Klebsiella pneumoniae species complex plasmids shows a highly diverse and ecologically adaptable plasmidome.
Microbial genomics, 12(2):.
Plasmids play a pivotal role in the horizontal gene transfer (HGT) of antimicrobial resistance (AMR) and virulence determinants among bacteria. Members of the Klebsiella pneumoniae species complex (KpSC) can colonize humans, animals and various environments and frequently cause nosocomial and community-acquired infections in humans. While plasmid-borne AMR genes are prevalent in clinical strains, the diversity, distribution and association of plasmids encoding AMR and virulence across ecological niches remain poorly characterized. Understanding the traits governing successful plasmid transmission within and between ecological niches is critical for developing effective AMR prevention strategies. Here, we identify ecological and structural factors shaping plasmid persistence and dissemination. We analysed the plasmidome (i.e. total genetic content attributable to plasmids) of 578 whole-genome sequenced KpSC isolates collected in Norway between 2001 and 2020 from human (n=453), animal (n=102) and marine (n=23) sources. Plasmids from complete hybrid assemblies were annotated and clustered to evaluate the plasmid diversity and content across niches. Additionally, the representativeness of this plasmid collection was determined by clustering with a global collection of 8,656 circularized KpSC plasmids. In total, 1,415 circularized plasmids were identified and grouped according to rearrangement distance using Pling, resulting in 130 clusters (≥2 plasmids each), of which 36% (n=47) contained plasmids from more than one niche. The plasmids exhibited significant diversity, as 37% (n=524) remained singletons after clustering. AMR and virulence genes existed across diverse clusters and singletons but predominantly resided on 120-250 kbp conjugative or mobilizable plasmids harbouring various transposable elements. Human isolates carried higher overall plasmid burdens and harboured most AMR-encoding plasmids, while animal isolates were significantly enriched for virulence plasmids (P<0.001), largely due to iuc3 plasmids in pigs. Plasmids from human, animal and marine isolates formed shared genetic clusters spanning ecological boundaries, revealing the existence of widely distributed backbones already primed for AMR gene acquisition. The extensive diversity of KpSC plasmids highlights the dynamic nature of plasmid evolution, driven by HGT and selective pressures. The presence of variable clusters, marked by high genetic diversity, indicates a dynamic plasmidome capable of rapid adaptation to environmental pressures through the acquisition and rearrangement of accessory genes.
Additional Links: PMID-41712274
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41712274,
year = {2026},
author = {Winkler, MA and Hetland, MAK and Kaspersen, HP and Bakksjø, RJ and Bernhoff, E and Fostervold, A and Hawkey, J and Lunestad, BT and Marathe, NP and Raffelsberger, N and Samuelsen, Ø and Sunde, M and Sundsfjord, A and Lam, MMC and Löhr, IH},
title = {A One Health study of Klebsiella pneumoniae species complex plasmids shows a highly diverse and ecologically adaptable plasmidome.},
journal = {Microbial genomics},
volume = {12},
number = {2},
pages = {},
doi = {10.1099/mgen.0.001629},
pmid = {41712274},
issn = {2057-5858},
mesh = {*Klebsiella pneumoniae/genetics/pathogenicity/isolation & purification/classification ; *Plasmids/genetics ; Humans ; Animals ; Gene Transfer, Horizontal ; Klebsiella Infections/microbiology ; Whole Genome Sequencing ; One Health ; Virulence/genetics ; Genetic Variation ; Norway ; Genome, Bacterial ; },
abstract = {Plasmids play a pivotal role in the horizontal gene transfer (HGT) of antimicrobial resistance (AMR) and virulence determinants among bacteria. Members of the Klebsiella pneumoniae species complex (KpSC) can colonize humans, animals and various environments and frequently cause nosocomial and community-acquired infections in humans. While plasmid-borne AMR genes are prevalent in clinical strains, the diversity, distribution and association of plasmids encoding AMR and virulence across ecological niches remain poorly characterized. Understanding the traits governing successful plasmid transmission within and between ecological niches is critical for developing effective AMR prevention strategies. Here, we identify ecological and structural factors shaping plasmid persistence and dissemination. We analysed the plasmidome (i.e. total genetic content attributable to plasmids) of 578 whole-genome sequenced KpSC isolates collected in Norway between 2001 and 2020 from human (n=453), animal (n=102) and marine (n=23) sources. Plasmids from complete hybrid assemblies were annotated and clustered to evaluate the plasmid diversity and content across niches. Additionally, the representativeness of this plasmid collection was determined by clustering with a global collection of 8,656 circularized KpSC plasmids. In total, 1,415 circularized plasmids were identified and grouped according to rearrangement distance using Pling, resulting in 130 clusters (≥2 plasmids each), of which 36% (n=47) contained plasmids from more than one niche. The plasmids exhibited significant diversity, as 37% (n=524) remained singletons after clustering. AMR and virulence genes existed across diverse clusters and singletons but predominantly resided on 120-250 kbp conjugative or mobilizable plasmids harbouring various transposable elements. Human isolates carried higher overall plasmid burdens and harboured most AMR-encoding plasmids, while animal isolates were significantly enriched for virulence plasmids (P<0.001), largely due to iuc3 plasmids in pigs. Plasmids from human, animal and marine isolates formed shared genetic clusters spanning ecological boundaries, revealing the existence of widely distributed backbones already primed for AMR gene acquisition. The extensive diversity of KpSC plasmids highlights the dynamic nature of plasmid evolution, driven by HGT and selective pressures. The presence of variable clusters, marked by high genetic diversity, indicates a dynamic plasmidome capable of rapid adaptation to environmental pressures through the acquisition and rearrangement of accessory genes.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Klebsiella pneumoniae/genetics/pathogenicity/isolation & purification/classification
*Plasmids/genetics
Humans
Animals
Gene Transfer, Horizontal
Klebsiella Infections/microbiology
Whole Genome Sequencing
One Health
Virulence/genetics
Genetic Variation
Norway
Genome, Bacterial
RevDate: 2026-02-19
CmpDate: 2026-02-19
A One Health Perspective on the Plasmid Backbone Preference and Evolutionary Adaptation of tmexCD-toprJ in Klebsiella spp.
Infection and drug resistance, 19:585632.
BACKGROUND: Antimicrobial resistance (AMR) poses a critical One Health challenge, linking human, animal, and environmental health through the movement of multidrug-resistant (MDR) bacteria and resistance determinants. The tmexCD-toprJ gene cluster, an efflux pump conferring high-level resistance to tigecycline and eravacycline. However, its plasmid backbone preferences and evolutionary trajectories in Klebsiella spp. remain insufficiently characterized.
METHODS: This study investigated the plasmid backbone preference and evolutionary characteristics of tmexCD-toprJ-harboring plasmids in Klebsiella spp. using whole-genome sequencing of three clinical strains carrying tmexCD-toprJ collected from 2018 to 2023. Conjugation assays, comparative genomics, and global epidemiological analysis were performed to assess plasmid mobility, genetic context, and evolutionary direction under the One Health framework.
RESULTS: All three isolates (K7, K36, and K307) exhibited MDR and harbored major resistance genes, including blaIMP-4, mcr-1.1, and blaNDM-1 , respectively. The plasmid from K36 was transferable to EC600 (frequency, 10[-7]), confirming cross-species mobility. Global database analysis revealed that tmexCD-toprJ-positive Klebsiella spp. isolates (n=92) originated mainly from humans (59.8%), followed by animals (37.0%) and environments (3.3%). Phylogenetic and plasmid analyses the tmexCD1-toprJ1 variant was mainly associated with these hybrid plasmids, frequently co-localizing with sul1, qnrB, and strA/B to form stable "tigecycline-aminoglycoside-sulfonamide" co-resistance modules. In contrast, tmexCD2-toprJ2 was more often inserted into classical resistant plasmids.
CONCLUSION: These findings demonstrate that tmexCD-toprJ has evolved as a highly mobile resistance determinant within Klebsiella spp. disseminating across the human-animal-environment interface via hybrid plasmids and horizontal gene transfer. This underscores the urgent need for integrated One Health surveillance and containment strategies to mitigate plasmid-mediated multidrug resistance and its global public health impact.
Additional Links: PMID-41710376
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41710376,
year = {2026},
author = {Jiang, X and Liu, F and Chai, J and Li, X and Li, Y and Fu, S and Li, Y},
title = {A One Health Perspective on the Plasmid Backbone Preference and Evolutionary Adaptation of tmexCD-toprJ in Klebsiella spp.},
journal = {Infection and drug resistance},
volume = {19},
number = {},
pages = {585632},
pmid = {41710376},
issn = {1178-6973},
abstract = {BACKGROUND: Antimicrobial resistance (AMR) poses a critical One Health challenge, linking human, animal, and environmental health through the movement of multidrug-resistant (MDR) bacteria and resistance determinants. The tmexCD-toprJ gene cluster, an efflux pump conferring high-level resistance to tigecycline and eravacycline. However, its plasmid backbone preferences and evolutionary trajectories in Klebsiella spp. remain insufficiently characterized.
METHODS: This study investigated the plasmid backbone preference and evolutionary characteristics of tmexCD-toprJ-harboring plasmids in Klebsiella spp. using whole-genome sequencing of three clinical strains carrying tmexCD-toprJ collected from 2018 to 2023. Conjugation assays, comparative genomics, and global epidemiological analysis were performed to assess plasmid mobility, genetic context, and evolutionary direction under the One Health framework.
RESULTS: All three isolates (K7, K36, and K307) exhibited MDR and harbored major resistance genes, including blaIMP-4, mcr-1.1, and blaNDM-1 , respectively. The plasmid from K36 was transferable to EC600 (frequency, 10[-7]), confirming cross-species mobility. Global database analysis revealed that tmexCD-toprJ-positive Klebsiella spp. isolates (n=92) originated mainly from humans (59.8%), followed by animals (37.0%) and environments (3.3%). Phylogenetic and plasmid analyses the tmexCD1-toprJ1 variant was mainly associated with these hybrid plasmids, frequently co-localizing with sul1, qnrB, and strA/B to form stable "tigecycline-aminoglycoside-sulfonamide" co-resistance modules. In contrast, tmexCD2-toprJ2 was more often inserted into classical resistant plasmids.
CONCLUSION: These findings demonstrate that tmexCD-toprJ has evolved as a highly mobile resistance determinant within Klebsiella spp. disseminating across the human-animal-environment interface via hybrid plasmids and horizontal gene transfer. This underscores the urgent need for integrated One Health surveillance and containment strategies to mitigate plasmid-mediated multidrug resistance and its global public health impact.},
}
RevDate: 2026-02-18
Antibiotics or Heavy Metals in Livestock Wastewater: Which One Is the Main Driver for the Development and Spread of Antibiotic Resistance under Coexposure?.
Environmental science & technology [Epub ahead of print].
Antibiotics and heavy metals are widely used in livestock farming to promote animal health and growth, leading to their frequent co-occurrence as contaminants in livestock wastewater. However, their relative contributions to shaping the antibiotic resistome in treatment systems remain unclear. In this study, we simulated an aerobic activated sludge process treating livestock wastewater containing enrofloxacin and heavy metals (Cu[2+] and Zn[2+]) to evaluate the development of antibiotic resistance using metagenomic and metatranscriptomic approaches. We observed a diverse and transcriptionally active resistome with over half of the detected antibiotic resistance genes (ARGs) showing expression. ARG profiles under coexposure to enrofloxacin and heavy metals more closely resembled those under heavy metal exposure alone than those under enrofloxacin exposure alone. Zn[2+] exposure resulted in the highest absolute ARG abundance, nearly double that of the control group. Both enrofloxacin and heavy metals significantly altered the abundance and phylogenetic composition of the antibiotic-resistant bacteria (ARB). The exposure to Zn[2+] enhanced the relative abundance and expression level of both metal resistance genes (MRGs)-carrying ARB and the ARGs-carrying plasmids. Phylogenetic analysis of ARG flanking sequences revealed high homology across various genetic contexts. Among mobile genetic elements, plasmids had a greater influence on ARG profiles than did phages or integrative and conjugative elements (ICEs). Transcriptional profiles of microbial physiological adaptations suggested that modulation of cell membrane permeability, promotion of conjugative transfer, and formation of biofilm might play roles in enhancing antibiotic resistance. These findings suggest at environmentally relevant concentrations, heavy metals such as Zn[2+] may present a stronger selective pressure than enrofloxacin for the propagation of antibiotic resistance in aerobic activated sludge process treating livestock wastewater.
Additional Links: PMID-41708296
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41708296,
year = {2026},
author = {Huang, J and Zhang, J and Liang, H and Fang, P and Tang, A and Klümper, U and Guo, J and Berendonk, TU and Honda, R and Lin, L and Li, X and Li, B},
title = {Antibiotics or Heavy Metals in Livestock Wastewater: Which One Is the Main Driver for the Development and Spread of Antibiotic Resistance under Coexposure?.},
journal = {Environmental science & technology},
volume = {},
number = {},
pages = {},
doi = {10.1021/acs.est.5c06042},
pmid = {41708296},
issn = {1520-5851},
abstract = {Antibiotics and heavy metals are widely used in livestock farming to promote animal health and growth, leading to their frequent co-occurrence as contaminants in livestock wastewater. However, their relative contributions to shaping the antibiotic resistome in treatment systems remain unclear. In this study, we simulated an aerobic activated sludge process treating livestock wastewater containing enrofloxacin and heavy metals (Cu[2+] and Zn[2+]) to evaluate the development of antibiotic resistance using metagenomic and metatranscriptomic approaches. We observed a diverse and transcriptionally active resistome with over half of the detected antibiotic resistance genes (ARGs) showing expression. ARG profiles under coexposure to enrofloxacin and heavy metals more closely resembled those under heavy metal exposure alone than those under enrofloxacin exposure alone. Zn[2+] exposure resulted in the highest absolute ARG abundance, nearly double that of the control group. Both enrofloxacin and heavy metals significantly altered the abundance and phylogenetic composition of the antibiotic-resistant bacteria (ARB). The exposure to Zn[2+] enhanced the relative abundance and expression level of both metal resistance genes (MRGs)-carrying ARB and the ARGs-carrying plasmids. Phylogenetic analysis of ARG flanking sequences revealed high homology across various genetic contexts. Among mobile genetic elements, plasmids had a greater influence on ARG profiles than did phages or integrative and conjugative elements (ICEs). Transcriptional profiles of microbial physiological adaptations suggested that modulation of cell membrane permeability, promotion of conjugative transfer, and formation of biofilm might play roles in enhancing antibiotic resistance. These findings suggest at environmentally relevant concentrations, heavy metals such as Zn[2+] may present a stronger selective pressure than enrofloxacin for the propagation of antibiotic resistance in aerobic activated sludge process treating livestock wastewater.},
}
RevDate: 2026-02-18
Toxin-antitoxin systems propagate through addictive selection during bacterial chromosome-plasmid conflicts.
FEMS microbiology letters pii:8489719 [Epub ahead of print].
Plasmids are obligate genetic parasites that significantly influence bacterial host adaptation, ecology, and clinically relevant traits such as antibiotic resistance. They persist within host populations primarily through self-maintenance mechanisms, most notably Toxin-Antitoxin (TA) systems, which are autoregulated poison-antidote operons mediating genomic conflict. Plasmid-encoded TAs act as "addiction modules," promoting plasmid stability via post-segregational killing of daughter cells that fail to inherit the plasmid. However, the widespread and abundant presence of TAs on bacterial chromosomes remains an evolutionary puzzle. We conducted comprehensive bioinformatics analyses of 11,000 bacterial chromosomes and 1,300 plasmids, focusing on Type II TAs in Escherichia and Shigella species, to elucidate their prevalence, distribution, and ecological significance. Our results reveal distinct horizontal gene transfer patterns and strongly support the anti-addiction hypothesis, which posits that chromosomal TAs protect host cells by neutralizing TA-plasmid addiction effects. This neutralization allows for plasmid loss without the toxin-mediated lethal consequences, resulting in a pattern of mutual exclusivity between identical chromosomal and plasmid TAs. This study reinforces the view that chromosomal Type II toxin-antitoxin systems play a significant role in counteracting addiction processes within bacterial chromosomes.
Additional Links: PMID-41707201
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41707201,
year = {2026},
author = {Sudhakari, PA and Ramisetty, BCM},
title = {Toxin-antitoxin systems propagate through addictive selection during bacterial chromosome-plasmid conflicts.},
journal = {FEMS microbiology letters},
volume = {},
number = {},
pages = {},
doi = {10.1093/femsle/fnag021},
pmid = {41707201},
issn = {1574-6968},
abstract = {Plasmids are obligate genetic parasites that significantly influence bacterial host adaptation, ecology, and clinically relevant traits such as antibiotic resistance. They persist within host populations primarily through self-maintenance mechanisms, most notably Toxin-Antitoxin (TA) systems, which are autoregulated poison-antidote operons mediating genomic conflict. Plasmid-encoded TAs act as "addiction modules," promoting plasmid stability via post-segregational killing of daughter cells that fail to inherit the plasmid. However, the widespread and abundant presence of TAs on bacterial chromosomes remains an evolutionary puzzle. We conducted comprehensive bioinformatics analyses of 11,000 bacterial chromosomes and 1,300 plasmids, focusing on Type II TAs in Escherichia and Shigella species, to elucidate their prevalence, distribution, and ecological significance. Our results reveal distinct horizontal gene transfer patterns and strongly support the anti-addiction hypothesis, which posits that chromosomal TAs protect host cells by neutralizing TA-plasmid addiction effects. This neutralization allows for plasmid loss without the toxin-mediated lethal consequences, resulting in a pattern of mutual exclusivity between identical chromosomal and plasmid TAs. This study reinforces the view that chromosomal Type II toxin-antitoxin systems play a significant role in counteracting addiction processes within bacterial chromosomes.},
}
RevDate: 2026-02-18
CmpDate: 2026-02-18
Emergence of a KL239-OCL6-ST63 Carbapenem-Resistant Acinetobacter pittii Strain, Co-carrying blaNDM-1 and blaOXA-500.
Current microbiology, 83(4):181.
To characterize the genomic features, antimicrobial resistance mechanisms, and biological characteristics of a carbapenem-resistant Acinetobacter pittii strain co-harboring plasmid-borne blaNDM-1 and chromosomally located blaOXA-500. An A. pittii strain (L802) was isolated from an intestinal sample of a diarrhea outpatient in Hangzhou, Zhejiang Province, China. Whole-genome sequencing was performed using Illumina and Oxford Nanopore platforms, followed by comprehensive bioinformatics analysis. The localization of blaNDM-1 was determined by S1-PFGE and Southern blotting. Horizontal gene transfer potential was evaluated by conjugation and electrotransformation assays. Antimicrobial susceptibility testing, biofilm formation assays, virulence evaluation using a Galleria mellonella infection model, scanning electron microscopy, phylogenetic analysis, and RT-qPCR analysis of resistance gene expression under carbapenem induction were conducted. Strain L802 was identified as A. pittii ST63 and exhibited high-level resistance to carbapenems and multiple cephalosporins, while remaining susceptible to polymyxin B and tigecycline. Whole-genome analysis revealed a 3.86 Mb circular chromosome and four plasmids. The blaNDM-1 gene was located on a ~ 41 kb IncR-type plasmid (pL802-NDM-1) together with aph(3')-VI, sharing 99-100% sequence identity with plasmids from diverse Enterobacteriaceae species. Conjugation assays failed to yield transconjugants; however, electrotransformation confirmed that the blaNDM-1-carrying plasmid could be introduced into Escherichia coli DH5α under laboratory conditions. Importantly, blaOXA-500 was located on the chromosome, representing a rare genetic configuration that may contribute to enhanced stability compared with plasmid-borne resistance genes. Phenotypic assays showed weak biofilm formation and low virulence in the Galleria mellonella model. Phylogenetic analysis indicated that L802 clustered closely with other A. pittii strains isolated in China, suggesting possible regional dissemination. This study reports, for the first time in Zhejiang, China, an A. pittii strain co-harboring plasmid-borne blaNDM-1 and chromosomally located blaOXA-500. The coexistence of mobile and chromosomally encoded carbapenemase genes highlights a concerning resistance strategy and underscores the need for continuous surveillance and infection control measures against emerging multidrug-resistant Acinetobacter species.
Additional Links: PMID-41706200
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41706200,
year = {2026},
author = {Zhou, D and Fan, J and Zhang, D and Ma, X and Li, Y and Zhang, X and Zheng, S and Hou, Q and Li, S and Li, G and Han, H},
title = {Emergence of a KL239-OCL6-ST63 Carbapenem-Resistant Acinetobacter pittii Strain, Co-carrying blaNDM-1 and blaOXA-500.},
journal = {Current microbiology},
volume = {83},
number = {4},
pages = {181},
pmid = {41706200},
issn = {1432-0991},
support = {no.202410201105//the Jilin Province's Training Program of Innovation and Entrepreneurship for Undergraduates/ ; },
mesh = {*beta-Lactamases/genetics/metabolism ; *Acinetobacter/genetics/drug effects/isolation & purification/enzymology/classification ; *Carbapenems/pharmacology ; *Anti-Bacterial Agents/pharmacology ; Animals ; Plasmids/genetics ; *Acinetobacter Infections/microbiology ; Phylogeny ; Humans ; China ; *Bacterial Proteins/genetics/metabolism ; Microbial Sensitivity Tests ; Gene Transfer, Horizontal ; Genome, Bacterial ; Moths/microbiology ; Whole Genome Sequencing ; Drug Resistance, Multiple, Bacterial/genetics ; },
abstract = {To characterize the genomic features, antimicrobial resistance mechanisms, and biological characteristics of a carbapenem-resistant Acinetobacter pittii strain co-harboring plasmid-borne blaNDM-1 and chromosomally located blaOXA-500. An A. pittii strain (L802) was isolated from an intestinal sample of a diarrhea outpatient in Hangzhou, Zhejiang Province, China. Whole-genome sequencing was performed using Illumina and Oxford Nanopore platforms, followed by comprehensive bioinformatics analysis. The localization of blaNDM-1 was determined by S1-PFGE and Southern blotting. Horizontal gene transfer potential was evaluated by conjugation and electrotransformation assays. Antimicrobial susceptibility testing, biofilm formation assays, virulence evaluation using a Galleria mellonella infection model, scanning electron microscopy, phylogenetic analysis, and RT-qPCR analysis of resistance gene expression under carbapenem induction were conducted. Strain L802 was identified as A. pittii ST63 and exhibited high-level resistance to carbapenems and multiple cephalosporins, while remaining susceptible to polymyxin B and tigecycline. Whole-genome analysis revealed a 3.86 Mb circular chromosome and four plasmids. The blaNDM-1 gene was located on a ~ 41 kb IncR-type plasmid (pL802-NDM-1) together with aph(3')-VI, sharing 99-100% sequence identity with plasmids from diverse Enterobacteriaceae species. Conjugation assays failed to yield transconjugants; however, electrotransformation confirmed that the blaNDM-1-carrying plasmid could be introduced into Escherichia coli DH5α under laboratory conditions. Importantly, blaOXA-500 was located on the chromosome, representing a rare genetic configuration that may contribute to enhanced stability compared with plasmid-borne resistance genes. Phenotypic assays showed weak biofilm formation and low virulence in the Galleria mellonella model. Phylogenetic analysis indicated that L802 clustered closely with other A. pittii strains isolated in China, suggesting possible regional dissemination. This study reports, for the first time in Zhejiang, China, an A. pittii strain co-harboring plasmid-borne blaNDM-1 and chromosomally located blaOXA-500. The coexistence of mobile and chromosomally encoded carbapenemase genes highlights a concerning resistance strategy and underscores the need for continuous surveillance and infection control measures against emerging multidrug-resistant Acinetobacter species.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*beta-Lactamases/genetics/metabolism
*Acinetobacter/genetics/drug effects/isolation & purification/enzymology/classification
*Carbapenems/pharmacology
*Anti-Bacterial Agents/pharmacology
Animals
Plasmids/genetics
*Acinetobacter Infections/microbiology
Phylogeny
Humans
China
*Bacterial Proteins/genetics/metabolism
Microbial Sensitivity Tests
Gene Transfer, Horizontal
Genome, Bacterial
Moths/microbiology
Whole Genome Sequencing
Drug Resistance, Multiple, Bacterial/genetics
RevDate: 2026-02-18
CmpDate: 2026-02-18
Chromosomal dif sites and associated modules identified in Acinetobacter sp. drive the horizontal transfer of antibiotic resistance.
Frontiers in microbiology, 16:1708097.
INTRODUCTION: Modules containing antibiotic resistance genes (ARGs) flanked by Xer site-specific recombination sites have been identified in Acinetobacter plasmids and are considered mobile genetic elements (MGEs) that facilitate horizontal gene transfer via the XerCD site-specific recombination (XerCD SSR) system. Although additional dif-like sites have been identified on the Acinetobacter chromosome beyond the main locus, it remains unclear whether these sites are associated with chromosomal dif modules.
METHODS: MacConkey agar plates supplemented with meropenem were used to isolate the resistant strain. Whole-genome sequencing (WGS) was performed on the Oxford Nanopore platform, and the bacterial species was identified using Average Nucleotide Identity (ANI) and digital DNA-DNA hybridization (dDDH). Antimicrobial susceptibility testing was performed against 18 antibiotics. Identification of dif and pdif sites was performed using BLAST tools.
RESULTS: This study identified numerous Xer modules containing resistance genes, IS elements, and other functional genes within the chromosome and plasmid of strain M10 (Acinetobacter sp.) isolated from a farmer at a cattle farm in Guangxi, China. Genome analysis and antimicrobial susceptibility testing confirm the association between these modules carrying resistance genes and resistant phenotypes. Chromosomal dif sites and associated dif modules in the strain were highly similar (sequence identity >99%) to plasmid-carried pdif sites and associated pdif modules in the public database. These suggest that additional chromosomal dif-like sites facilitate dif module formation, and that gene flow occurs between the chromosomes and plasmids of Acinetobacter. Furthermore, most Xer sites clustered to form a linear multi-module array, termed chromosomal dif module island and plasmid-borne pdif module island. Similar configurations were frequently observed in public Acinetobacter plasmid genomes.
DISCUSSION: Additional dif-like sites are present in Acinetobacter chromosomes, which are unlikely to play a function in chromosomal dimer resolution, and the modules they form are functionally similar to pdif modules, both of which play an important role in horizontal gene transfer.
Additional Links: PMID-41704853
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41704853,
year = {2025},
author = {Wang, Q and Wang, W and Qiu, Y and Dai, G and Li, B and Zhou, Y and Bai, Y and Zhang, J},
title = {Chromosomal dif sites and associated modules identified in Acinetobacter sp. drive the horizontal transfer of antibiotic resistance.},
journal = {Frontiers in microbiology},
volume = {16},
number = {},
pages = {1708097},
pmid = {41704853},
issn = {1664-302X},
abstract = {INTRODUCTION: Modules containing antibiotic resistance genes (ARGs) flanked by Xer site-specific recombination sites have been identified in Acinetobacter plasmids and are considered mobile genetic elements (MGEs) that facilitate horizontal gene transfer via the XerCD site-specific recombination (XerCD SSR) system. Although additional dif-like sites have been identified on the Acinetobacter chromosome beyond the main locus, it remains unclear whether these sites are associated with chromosomal dif modules.
METHODS: MacConkey agar plates supplemented with meropenem were used to isolate the resistant strain. Whole-genome sequencing (WGS) was performed on the Oxford Nanopore platform, and the bacterial species was identified using Average Nucleotide Identity (ANI) and digital DNA-DNA hybridization (dDDH). Antimicrobial susceptibility testing was performed against 18 antibiotics. Identification of dif and pdif sites was performed using BLAST tools.
RESULTS: This study identified numerous Xer modules containing resistance genes, IS elements, and other functional genes within the chromosome and plasmid of strain M10 (Acinetobacter sp.) isolated from a farmer at a cattle farm in Guangxi, China. Genome analysis and antimicrobial susceptibility testing confirm the association between these modules carrying resistance genes and resistant phenotypes. Chromosomal dif sites and associated dif modules in the strain were highly similar (sequence identity >99%) to plasmid-carried pdif sites and associated pdif modules in the public database. These suggest that additional chromosomal dif-like sites facilitate dif module formation, and that gene flow occurs between the chromosomes and plasmids of Acinetobacter. Furthermore, most Xer sites clustered to form a linear multi-module array, termed chromosomal dif module island and plasmid-borne pdif module island. Similar configurations were frequently observed in public Acinetobacter plasmid genomes.
DISCUSSION: Additional dif-like sites are present in Acinetobacter chromosomes, which are unlikely to play a function in chromosomal dimer resolution, and the modules they form are functionally similar to pdif modules, both of which play an important role in horizontal gene transfer.},
}
RevDate: 2026-02-17
Emerging zoonotic risks: whole-genome sequencing reveals antimicrobial resistance and genomic diversity in Providencia stuartii isolated from broiler chickens in Noakhali, Bangladesh.
Poultry science, 105(5):106602 pii:S0032-5791(26)00229-4 [Epub ahead of print].
Providencia stuartii is emerging as an Extensively Drug-Resistant (XDR) pathogen commonly found in animals, insects, and in burned and immunocompromised conditions. The misuse of antibiotics in poultry feed causes the emergence of XDR bacteria in the poultry industry. The knowledge of zoonotic transmissibility of poultry-derived P. stuartii remains elusive in Noakhali, Bangladesh. Poultry fecal and rectal swab samples were collected from selected farms in Noakhali, Bangladesh. Bacterial isolation and identification were performed using MacConkey agar, biochemical tests, and 16S rRNA Sanger sequencing. Antimicrobial susceptibility was assessed by the Kirby-Bauer disk diffusion method, and isolates with high multiple antibiotic resistance (MAR) indices were selected for whole-genome sequencing (WGS). Quality control, genome assembly, annotation, gene identification, pan-genome analysis, pathogenicity profiling, and comparative proteome analyses were subsequently conducted. Antibiogram analysis showed that ps_nstu_001 and ps_nstu_002 were resistant to 17 and 13 tested antibiotics, respectively. Furthermore, whole-genome sequencing revealed that both strains harbored resistance determinants to aminoglycosides, tetracyclines, sulfonamides, cephalosporins, β-lactams, and carbapenems. Additionally, mobile genetic elements (MGEs) and plasmids were identified, which represent the horizontal gene transfer capability. Moreover, pangenome analysis revealed ongoing gene acquisition and substantial genomic diversity among the isolates. The isolate ps_nstu_001 was identified as a putative human pathogen and clustered closely with a clinical strain isolated in the United States. In contrast, ps_nstu_002 was predicted to be a non-human pathogen; however, it exhibited a clear evolutionary relationship with a clinical isolate obtained from a diarrheal patient in Bangladesh, suggesting potential pathogenic relevance. Global pathogenic potential of the studied strains and key proteomic similarities between pathogenic and non-pathogenic strains revealed by pathogenicity profiling and proteome comparison. To conclude, these XDR isolates indicate the potential for zoonotic transmission and the spread of resistant genes to other animals, posing a significant public health risk.
Additional Links: PMID-41702340
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41702340,
year = {2026},
author = {Asha, IJ and Gupta, SD and Munim, MA and Akter, NN and Tamanna, S and Rahman, A and Imran, A and Das, SC and Hossain, MM and Islam, MM and Barman, DN},
title = {Emerging zoonotic risks: whole-genome sequencing reveals antimicrobial resistance and genomic diversity in Providencia stuartii isolated from broiler chickens in Noakhali, Bangladesh.},
journal = {Poultry science},
volume = {105},
number = {5},
pages = {106602},
doi = {10.1016/j.psj.2026.106602},
pmid = {41702340},
issn = {1525-3171},
abstract = {Providencia stuartii is emerging as an Extensively Drug-Resistant (XDR) pathogen commonly found in animals, insects, and in burned and immunocompromised conditions. The misuse of antibiotics in poultry feed causes the emergence of XDR bacteria in the poultry industry. The knowledge of zoonotic transmissibility of poultry-derived P. stuartii remains elusive in Noakhali, Bangladesh. Poultry fecal and rectal swab samples were collected from selected farms in Noakhali, Bangladesh. Bacterial isolation and identification were performed using MacConkey agar, biochemical tests, and 16S rRNA Sanger sequencing. Antimicrobial susceptibility was assessed by the Kirby-Bauer disk diffusion method, and isolates with high multiple antibiotic resistance (MAR) indices were selected for whole-genome sequencing (WGS). Quality control, genome assembly, annotation, gene identification, pan-genome analysis, pathogenicity profiling, and comparative proteome analyses were subsequently conducted. Antibiogram analysis showed that ps_nstu_001 and ps_nstu_002 were resistant to 17 and 13 tested antibiotics, respectively. Furthermore, whole-genome sequencing revealed that both strains harbored resistance determinants to aminoglycosides, tetracyclines, sulfonamides, cephalosporins, β-lactams, and carbapenems. Additionally, mobile genetic elements (MGEs) and plasmids were identified, which represent the horizontal gene transfer capability. Moreover, pangenome analysis revealed ongoing gene acquisition and substantial genomic diversity among the isolates. The isolate ps_nstu_001 was identified as a putative human pathogen and clustered closely with a clinical strain isolated in the United States. In contrast, ps_nstu_002 was predicted to be a non-human pathogen; however, it exhibited a clear evolutionary relationship with a clinical isolate obtained from a diarrheal patient in Bangladesh, suggesting potential pathogenic relevance. Global pathogenic potential of the studied strains and key proteomic similarities between pathogenic and non-pathogenic strains revealed by pathogenicity profiling and proteome comparison. To conclude, these XDR isolates indicate the potential for zoonotic transmission and the spread of resistant genes to other animals, posing a significant public health risk.},
}
RevDate: 2026-02-17
Genetic Exchanges Shape the Evolutionary Diversification Among Shigella phages.
Journal of molecular evolution [Epub ahead of print].
Shigella is a genus of bacteria that is a prevalent cause of bacterial diarrhoea (i.e., shigellosis). Shigella bacteriophages are shaping bacterial fitness. Bacteriophages can carry genes that contribute to Shigella virulence and antibiotic resistance, and these genes are frequently found on mobile genetic elements (MGEs). Horizontal gene transfer (HGT) of these components is a major driver of bacterial evolution. A comprehensive genomic analysis of these bacteriophages is required to deepen understanding of candidate genes for MGEs and HGTs. Through genetic exchange, phages acquire novel genetic features that confer selective advantages. In this study, we identified the weighted gene repertoire relatedness (wGRR) metric. We associated it with the infecting host species andgenetic exchanges among Shigella phages using the weighted gene repertoire relatedness (wGRR) metric. We associated them with the infecting host species and phage lifestyles to examine evolutionary constraints among phages. We observed that HGTs can affect genes' GC content, which, in turn, influences amino acid usage, thereby shaping the amino acid usage of the resulting proteins. Host-range expansion is also observed among Shigella phages. However, we also noted that Shigella phages do not have the propensity for genetic transfer with dissimilar lifestyles. The gene pool of bacteriophages, due to horizontal transfer, can broaden their host range, making them more suitable for applications in phage therapy against antibiotic-resistant bacteria. Horizontal gene transfer can expand the bacteriophage gene pool, thereby increasing host range and making them more suitable for phage therapy against antibiotic-resistant bacteria. Overall, this study provides deeper insight into MGEs and HGTs among Shigella phages and their evolutionary significance for infectivity.
Additional Links: PMID-41701335
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41701335,
year = {2026},
author = {Chatterjee, J and Kayet, P and Ghosh, M and Dutta, S and Basak, S},
title = {Genetic Exchanges Shape the Evolutionary Diversification Among Shigella phages.},
journal = {Journal of molecular evolution},
volume = {},
number = {},
pages = {},
pmid = {41701335},
issn = {1432-1432},
support = {2021-10515//Indian Council of Medical Research/ ; },
abstract = {Shigella is a genus of bacteria that is a prevalent cause of bacterial diarrhoea (i.e., shigellosis). Shigella bacteriophages are shaping bacterial fitness. Bacteriophages can carry genes that contribute to Shigella virulence and antibiotic resistance, and these genes are frequently found on mobile genetic elements (MGEs). Horizontal gene transfer (HGT) of these components is a major driver of bacterial evolution. A comprehensive genomic analysis of these bacteriophages is required to deepen understanding of candidate genes for MGEs and HGTs. Through genetic exchange, phages acquire novel genetic features that confer selective advantages. In this study, we identified the weighted gene repertoire relatedness (wGRR) metric. We associated it with the infecting host species andgenetic exchanges among Shigella phages using the weighted gene repertoire relatedness (wGRR) metric. We associated them with the infecting host species and phage lifestyles to examine evolutionary constraints among phages. We observed that HGTs can affect genes' GC content, which, in turn, influences amino acid usage, thereby shaping the amino acid usage of the resulting proteins. Host-range expansion is also observed among Shigella phages. However, we also noted that Shigella phages do not have the propensity for genetic transfer with dissimilar lifestyles. The gene pool of bacteriophages, due to horizontal transfer, can broaden their host range, making them more suitable for applications in phage therapy against antibiotic-resistant bacteria. Horizontal gene transfer can expand the bacteriophage gene pool, thereby increasing host range and making them more suitable for phage therapy against antibiotic-resistant bacteria. Overall, this study provides deeper insight into MGEs and HGTs among Shigella phages and their evolutionary significance for infectivity.},
}
RevDate: 2026-02-17
CmpDate: 2026-02-17
Transposable elements as drivers of genome evolution in Drosophila virilis.
Nucleic acids research, 54(4):.
Transposable elements (TEs) drive genomic innovation, but their dynamics in non-model species remain unclear. Here, we integrated multi-omics data to explore TE dynamics in Drosophila virilis, an important model for repetitive DNA research. By combining computational predictions with manual curation, we identified 100 TE families and delineated three temporal waves of TE mobilization: recent activity, speciation-associated, and ancient invasions. TEs in D. virilis dynamically colonise both euchromatin and heterochromatin, suggesting heterochromatin is not solely a repository for degenerate repeats. While most TEs are widespread across strains, some exhibit strain-specific expansions, indicating varied activity and silencing. We found substantial evidence for horizontal transfer of TEs among close relatives, demonstrating that the D. virilis species group functions effectively as a TE "ecosystem", allowing for recurrent invasion, loss, and re-invasion of TE lineages across the group. Epigenetic profiling revealed that H3K9me3 spreading from TEs represses adjacent genes in a distance-dependent manner, influenced by insertion length and genomic context, affecting developmental and metabolic genes. We also discovered the first spontaneous polymorphic inversion in D. virilis linked to retrotransposons. Our findings illuminate TEs as drivers of genomic innovation, influencing gene regulation and evolutionary trajectories, providing a framework for studying TE dynamics across animal species.
Additional Links: PMID-41700089
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41700089,
year = {2026},
author = {Rezvykh, AP and Kulikova, DA and Zelentsova, ES and Protsenko, L and Bespalova, AV and Guseva, IO and Blumenstiel, JP and Evgen'ev, MB and Funikov, SY},
title = {Transposable elements as drivers of genome evolution in Drosophila virilis.},
journal = {Nucleic acids research},
volume = {54},
number = {4},
pages = {},
doi = {10.1093/nar/gkag139},
pmid = {41700089},
issn = {1362-4962},
support = {22-74-10050-P//Russian Science Foundation/ ; },
mesh = {Animals ; *Drosophila/genetics/classification ; *DNA Transposable Elements/genetics ; *Genome, Insect ; *Evolution, Molecular ; Heterochromatin/genetics ; Retroelements ; Epigenesis, Genetic ; Gene Transfer, Horizontal ; Euchromatin/genetics ; Phylogeny ; Histones/metabolism ; },
abstract = {Transposable elements (TEs) drive genomic innovation, but their dynamics in non-model species remain unclear. Here, we integrated multi-omics data to explore TE dynamics in Drosophila virilis, an important model for repetitive DNA research. By combining computational predictions with manual curation, we identified 100 TE families and delineated three temporal waves of TE mobilization: recent activity, speciation-associated, and ancient invasions. TEs in D. virilis dynamically colonise both euchromatin and heterochromatin, suggesting heterochromatin is not solely a repository for degenerate repeats. While most TEs are widespread across strains, some exhibit strain-specific expansions, indicating varied activity and silencing. We found substantial evidence for horizontal transfer of TEs among close relatives, demonstrating that the D. virilis species group functions effectively as a TE "ecosystem", allowing for recurrent invasion, loss, and re-invasion of TE lineages across the group. Epigenetic profiling revealed that H3K9me3 spreading from TEs represses adjacent genes in a distance-dependent manner, influenced by insertion length and genomic context, affecting developmental and metabolic genes. We also discovered the first spontaneous polymorphic inversion in D. virilis linked to retrotransposons. Our findings illuminate TEs as drivers of genomic innovation, influencing gene regulation and evolutionary trajectories, providing a framework for studying TE dynamics across animal species.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
Animals
*Drosophila/genetics/classification
*DNA Transposable Elements/genetics
*Genome, Insect
*Evolution, Molecular
Heterochromatin/genetics
Retroelements
Epigenesis, Genetic
Gene Transfer, Horizontal
Euchromatin/genetics
Phylogeny
Histones/metabolism
RevDate: 2026-02-17
CmpDate: 2026-02-17
Genomic Surveillance of Epiphytic Pseudomonas syringae Highlights Shared Reservoirs and Cross-Habitat Threats to Cherry Orchards and Nearby Woodland Plants.
Molecular plant pathology, 27(2):e70208.
Plant surfaces host diverse microbial communities acting as reservoirs for pathogenic lineages, yet the ecological dynamics and evolutionary consequences of such reservoirs remain underexplored. We conducted landscape-scale genomic surveillance of Pseudomonas syringae on symptomless leaves of cultivated cherry in orchards and wild plant species in adjacent woodlands across the UK, aiming to understand how phyllosphere populations contribute to the emergence of bacterial canker. Whole genome sequencing of 540 isolates collected over two years and across four regions revealed 10 diverse P. syringae phylogroups (PGs) on symptomless leaves. Both orchard and woodland environments harboured a similar range of PGs, but recovery frequency was very different. PG2d strains dominated cherry orchards, whereas PGs 2b and 13a were prevalent in woodlands. Certain PG2d subclades, recovered from both environments, caused disease on cultivated and wild cherry leaves. Additional strains were found to be pathogenic to Phaseolus bean pods. The pathogens of cherry were characterised by the presence of genes encoding the synthesis of the pathotoxin syringolin A and a subset of effector proteins including HopAW1, AvrRpm1 and HopAR1. Resolution of subclades within PG2d provided insights into the emergence of virulent epiphytic strains that have not yet reached the mostly northerly sampling sites but are threats to both cultivated and environmental Prunus spp. Fine-scale analysis of subclade PG2d-3 revealed potential divergence between orchard and woodland populations, with 49 genes exclusive to a woodland lineage. Thirty-eight of these genes were found within prophages, indicating the potential role of bacteriophage-mediated horizontal gene transfer in adaptation to non-agricultural reservoirs.
Additional Links: PMID-41699883
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41699883,
year = {2026},
author = {Zeng, Z and Mansfield, JW and Vadillo-Dieguez, A and Connell, J and Irvine, J and Hulin, MT and Frutos, FD and Rabiey, M and Grinberg, NF and Harrison, RJ and Xu, X and Jackson, RW},
title = {Genomic Surveillance of Epiphytic Pseudomonas syringae Highlights Shared Reservoirs and Cross-Habitat Threats to Cherry Orchards and Nearby Woodland Plants.},
journal = {Molecular plant pathology},
volume = {27},
number = {2},
pages = {e70208},
doi = {10.1111/mpp.70208},
pmid = {41699883},
issn = {1364-3703},
support = {BB/T010746/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom ; //Jabbs Foundation/ ; },
mesh = {*Pseudomonas syringae/genetics/pathogenicity ; *Plant Diseases/microbiology ; *Prunus avium/microbiology ; Phylogeny ; *Ecosystem ; Genome, Bacterial/genetics ; Plant Leaves/microbiology ; Genomics ; Forests ; },
abstract = {Plant surfaces host diverse microbial communities acting as reservoirs for pathogenic lineages, yet the ecological dynamics and evolutionary consequences of such reservoirs remain underexplored. We conducted landscape-scale genomic surveillance of Pseudomonas syringae on symptomless leaves of cultivated cherry in orchards and wild plant species in adjacent woodlands across the UK, aiming to understand how phyllosphere populations contribute to the emergence of bacterial canker. Whole genome sequencing of 540 isolates collected over two years and across four regions revealed 10 diverse P. syringae phylogroups (PGs) on symptomless leaves. Both orchard and woodland environments harboured a similar range of PGs, but recovery frequency was very different. PG2d strains dominated cherry orchards, whereas PGs 2b and 13a were prevalent in woodlands. Certain PG2d subclades, recovered from both environments, caused disease on cultivated and wild cherry leaves. Additional strains were found to be pathogenic to Phaseolus bean pods. The pathogens of cherry were characterised by the presence of genes encoding the synthesis of the pathotoxin syringolin A and a subset of effector proteins including HopAW1, AvrRpm1 and HopAR1. Resolution of subclades within PG2d provided insights into the emergence of virulent epiphytic strains that have not yet reached the mostly northerly sampling sites but are threats to both cultivated and environmental Prunus spp. Fine-scale analysis of subclade PG2d-3 revealed potential divergence between orchard and woodland populations, with 49 genes exclusive to a woodland lineage. Thirty-eight of these genes were found within prophages, indicating the potential role of bacteriophage-mediated horizontal gene transfer in adaptation to non-agricultural reservoirs.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Pseudomonas syringae/genetics/pathogenicity
*Plant Diseases/microbiology
*Prunus avium/microbiology
Phylogeny
*Ecosystem
Genome, Bacterial/genetics
Plant Leaves/microbiology
Genomics
Forests
RevDate: 2026-02-16
CmpDate: 2026-02-16
Functional characterization of macrolide esterase from cyanobacteria and their potential dissemination risk.
npj antimicrobials and resistance, 4(1):10.
The global dissemination of antibiotic resistance genes (ARGs) across diverse environments has emerged as a critical challenge to public health. As essential primary producers, Cyanobacteria colonize extreme and heterogeneous habitats, coexisting with gut microbiota in wastewater, marine ecosystems, and reservoirs, where they may potentiate the proliferation and transmission of ARGs under antibiotic selective pressures. In this study, three macrolide esterases (NOD-1, OCA-1, and OCB-1) of Cyanobacterial origin were identified through mining of local genomic repositories. These enzymes, classified as serine-dependent alpha/beta -hydrolases, were experimentally validated through antimicrobial susceptibility testing and zone of inhibition assays to inactivate specific 16-membered macrolide antibiotics. Comparative analysis of genomic regions flanking these resistance determinants revealed the presence of mobile genetic elements (MGEs) and co-localized multidrug resistance genes, strongly suggesting the likelihood of horizontal gene transfer (HGT) within Cyanobacterial populations. Such genetic mobility may exacerbate antibiotic resistance dissemination in aquatic ecosystems, underscoring the ecological risks posed by Cyanobacteria as reservoirs and vectors of ARGs.
Additional Links: PMID-41699255
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41699255,
year = {2026},
author = {Tao, H and Zhou, L and Zhou, Y and Wang, Y and Lv, H and Wang, T and Xu, C and Chu, Y and Wang, X and Song, T and Lin, J},
title = {Functional characterization of macrolide esterase from cyanobacteria and their potential dissemination risk.},
journal = {npj antimicrobials and resistance},
volume = {4},
number = {1},
pages = {10},
pmid = {41699255},
issn = {2731-8745},
support = {2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; 2023YFS0384;NO. FZBC2022003;ZSKHHZ [2021] No.320;Grant No.: 82372290;2023NSFSC1467//Sichuan key research and development program;Open Project Program of Irradiation Preservation Key Laboratory of Sichuan Province, Sichuan Institute of Atomic Energy;Zunyi Technology and Big data Bureau, Moutai institute Joint Science and Technology Research and Development Project;National Natural Science Foundation of China;Natural Science Foundation of Sichuan Province/ ; },
abstract = {The global dissemination of antibiotic resistance genes (ARGs) across diverse environments has emerged as a critical challenge to public health. As essential primary producers, Cyanobacteria colonize extreme and heterogeneous habitats, coexisting with gut microbiota in wastewater, marine ecosystems, and reservoirs, where they may potentiate the proliferation and transmission of ARGs under antibiotic selective pressures. In this study, three macrolide esterases (NOD-1, OCA-1, and OCB-1) of Cyanobacterial origin were identified through mining of local genomic repositories. These enzymes, classified as serine-dependent alpha/beta -hydrolases, were experimentally validated through antimicrobial susceptibility testing and zone of inhibition assays to inactivate specific 16-membered macrolide antibiotics. Comparative analysis of genomic regions flanking these resistance determinants revealed the presence of mobile genetic elements (MGEs) and co-localized multidrug resistance genes, strongly suggesting the likelihood of horizontal gene transfer (HGT) within Cyanobacterial populations. Such genetic mobility may exacerbate antibiotic resistance dissemination in aquatic ecosystems, underscoring the ecological risks posed by Cyanobacteria as reservoirs and vectors of ARGs.},
}
RevDate: 2026-02-16
Bio-based microplastics increase the horizontal transfer of antibiotic resistance genes in aquatic environments.
NanoImpact pii:S2452-0748(26)00003-0 [Epub ahead of print].
The role of microplastics as vectors for horizontal gene transfer (HGT) of antibiotic resistance genes (ARGs) is increasingly recognized. This study investigated whether bio-based microplastics, often promoted as environmentally friendly alternatives, exhibit similar or enhanced HGT potential compared to conventional plastics. We examined the HGT rates of the trimethoprim resistance gene (dfrA1) and tetracycline resistance gene (tetA), carried on a broad-host-range plasmid, among Escherichia coli (donor) and Vibrio parahaemolyticus, Pseudomonas sp., or a natural lake microbial community (recipients). Four bio-based polymer types-polylactic acid (PLA) granules, commercial PLA, high-density polyethylene (HDPE) granules, and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)- were compared with two conventional microplastics, polyethylene terephthalate (PET) and bottle-derived HDPE. The bio-based microplastics exhibited significantly higher HGT frequencies, with a 21-48-fold increase compared to control chitosan in single-strain experiments and a 13-fold increase within the lake microbial community. 16S rRNA amplicon sequencing revealed distinct bacterial community compositions colonizing different microplastic types in the lake water. The transconjugant communities, indicative of successful HGT events, were strongly influenced by microplastic type. While Nannocystis was generally dominant, the PLA (granule) microplastic exhibited a unique profile dominated by Candidatus Megaira and Niveispirillum. Additionally, Flavobacterium and Fluviicola were uniquely detected as transconjugants on HDPE (granule). These findings demonstrate that bioplastics have a significant influence on the selective enrichment of specific transconjugant genera, suggesting a prominent role of microplastics, particularly bio-plastics, in shaping ARG dissemination within complex microbial ecosystems. We recommend a comprehensive risk assessment of bio-based plastics, particularly their potential to enhance the spread of ARGs, before their widespread implementation in consumer products.
Additional Links: PMID-41698534
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41698534,
year = {2026},
author = {Jaffer, YD and Monikh, FA and Nguyen, NHA and Sevcu, A and Abdulkadir, N and Raha, J and Katsumiti, A and Bilbao, A and Altman, K and Grossart, HP},
title = {Bio-based microplastics increase the horizontal transfer of antibiotic resistance genes in aquatic environments.},
journal = {NanoImpact},
volume = {},
number = {},
pages = {100613},
doi = {10.1016/j.impact.2026.100613},
pmid = {41698534},
issn = {2452-0748},
abstract = {The role of microplastics as vectors for horizontal gene transfer (HGT) of antibiotic resistance genes (ARGs) is increasingly recognized. This study investigated whether bio-based microplastics, often promoted as environmentally friendly alternatives, exhibit similar or enhanced HGT potential compared to conventional plastics. We examined the HGT rates of the trimethoprim resistance gene (dfrA1) and tetracycline resistance gene (tetA), carried on a broad-host-range plasmid, among Escherichia coli (donor) and Vibrio parahaemolyticus, Pseudomonas sp., or a natural lake microbial community (recipients). Four bio-based polymer types-polylactic acid (PLA) granules, commercial PLA, high-density polyethylene (HDPE) granules, and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)- were compared with two conventional microplastics, polyethylene terephthalate (PET) and bottle-derived HDPE. The bio-based microplastics exhibited significantly higher HGT frequencies, with a 21-48-fold increase compared to control chitosan in single-strain experiments and a 13-fold increase within the lake microbial community. 16S rRNA amplicon sequencing revealed distinct bacterial community compositions colonizing different microplastic types in the lake water. The transconjugant communities, indicative of successful HGT events, were strongly influenced by microplastic type. While Nannocystis was generally dominant, the PLA (granule) microplastic exhibited a unique profile dominated by Candidatus Megaira and Niveispirillum. Additionally, Flavobacterium and Fluviicola were uniquely detected as transconjugants on HDPE (granule). These findings demonstrate that bioplastics have a significant influence on the selective enrichment of specific transconjugant genera, suggesting a prominent role of microplastics, particularly bio-plastics, in shaping ARG dissemination within complex microbial ecosystems. We recommend a comprehensive risk assessment of bio-based plastics, particularly their potential to enhance the spread of ARGs, before their widespread implementation in consumer products.},
}
RevDate: 2026-02-16
From Lake Victoria to the Tap: Antibiotic Resistance and Pathogenic Contamination of Kisumu City Water Supply and Wastewater Network.
Tropical medicine & international health : TM & IH [Epub ahead of print].
Waterborne diseases and antimicrobial resistance (AMR) pose mounting public health threats across sub-Saharan Africa, particularly in rapidly urbanising regions dependent on untreated or poorly treated surface waters. This study applied shotgun metagenomic sequencing to characterise microbial communities, virulence factors and antibiotic resistance genes (ARGs) in water samples collected from Lake Victoria, River Wigwa, Dunga Water Treatment Plant, Nyalenda Wastewater Stabilisation Ponds and the tap water outlet in post-treatment supply pipe in Kisumu city (Kenya). Bacterial taxa dominated all metagenomes, with 121 classes represented. Cyanobacteria, particularly Planktothrix, were highly abundant in lake and tap water, whereas wastewater and river samples exhibited greater taxonomic diversity. Major human pathogens, including Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii and Bacillus cereus/anthracis, were detected in nearly all samples, with unexpectedly high prevalence in tap water. Viral indicators of faecal contamination (adenoviruses, enteroviruses and torque teno viruses) corroborated widespread wastewater influence. Functional gene profiling revealed a rich resistome comprising aminoglycoside-modifying enzymes, β-lactamases, vancomycin-resistance operons and disinfectant-resistance determinants. The highest ARG and virulence gene frequencies occurred in tap and treatment-plant water, suggesting that incomplete disinfection and biofilm persistence promote the proliferation and exchange of ARGs between environmental and pathogenic taxa. In contrast, Lake Victoria water exhibited lower ARG abundance, reflecting natural self-purification processes. These findings underscore the inadequate water treatment and open wastewater systems create ecological 'hotspots' for ARG selection and horizontal gene transfer. Metagenomic surveillance integrated into One Health frameworks can enhance risk forecasting and guide interventions to mitigate AMR emergence and dissemination in freshwater systems serving over 35 million people across the Lake Victoria basin.
Additional Links: PMID-41697036
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41697036,
year = {2026},
author = {Reva, ON and Sifuna, A and Orata, F and Omolo, C and Iramiot, JS and Enright, MC and Mutshembele, A and Zhou, J and Shivoga, WA},
title = {From Lake Victoria to the Tap: Antibiotic Resistance and Pathogenic Contamination of Kisumu City Water Supply and Wastewater Network.},
journal = {Tropical medicine & international health : TM & IH},
volume = {},
number = {},
pages = {},
doi = {10.1111/tmi.70105},
pmid = {41697036},
issn = {1365-3156},
support = {GCRFNGR8\1143//UK Global Challenges Research Fund Networking/ ; NIHR163838//UK National Institute for Health and Care Research/ ; },
abstract = {Waterborne diseases and antimicrobial resistance (AMR) pose mounting public health threats across sub-Saharan Africa, particularly in rapidly urbanising regions dependent on untreated or poorly treated surface waters. This study applied shotgun metagenomic sequencing to characterise microbial communities, virulence factors and antibiotic resistance genes (ARGs) in water samples collected from Lake Victoria, River Wigwa, Dunga Water Treatment Plant, Nyalenda Wastewater Stabilisation Ponds and the tap water outlet in post-treatment supply pipe in Kisumu city (Kenya). Bacterial taxa dominated all metagenomes, with 121 classes represented. Cyanobacteria, particularly Planktothrix, were highly abundant in lake and tap water, whereas wastewater and river samples exhibited greater taxonomic diversity. Major human pathogens, including Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii and Bacillus cereus/anthracis, were detected in nearly all samples, with unexpectedly high prevalence in tap water. Viral indicators of faecal contamination (adenoviruses, enteroviruses and torque teno viruses) corroborated widespread wastewater influence. Functional gene profiling revealed a rich resistome comprising aminoglycoside-modifying enzymes, β-lactamases, vancomycin-resistance operons and disinfectant-resistance determinants. The highest ARG and virulence gene frequencies occurred in tap and treatment-plant water, suggesting that incomplete disinfection and biofilm persistence promote the proliferation and exchange of ARGs between environmental and pathogenic taxa. In contrast, Lake Victoria water exhibited lower ARG abundance, reflecting natural self-purification processes. These findings underscore the inadequate water treatment and open wastewater systems create ecological 'hotspots' for ARG selection and horizontal gene transfer. Metagenomic surveillance integrated into One Health frameworks can enhance risk forecasting and guide interventions to mitigate AMR emergence and dissemination in freshwater systems serving over 35 million people across the Lake Victoria basin.},
}
RevDate: 2026-02-15
Antibiotic sensitivity as a key Determinant: B. Subtilis Reshapes the Microecology to mitigate antibiotic resistance genes during composting.
Bioresource technology pii:S0960-8524(26)00303-2 [Epub ahead of print].
This study aimed to investigate the role of inoculant antibiotic susceptibility in controlling antibiotic resistance genes (ARGs) during aerobic composting. A systematic comparison was conducted using Bacillus subtilis strains (sensitive, S; resistant, R) to assess ARG dynamics, microbial community evolution, and the underlying ecological mechanisms. Results demonstrated that the sensitive strain significantly enhanced composting efficiency, achieving a higher and longer-lasting secondary thermophilic phase (58.4°C for 4 days) and superior maturity indices compared to the resistant strain. Crucially, the R strain counteracted the ARG-removal effect of high temperatures, increasing total ARG abundance by 28.40% by day 6 and resulting in a final ARG burden 2.74 times higher than the S treatment.Microecological mechanism analysis revealed that the sensitive strain fostered a specialized, modular microbial network with reduced niche breadth, enhancing community stability and functioning as a genetic firewall to restrict ARG dissemination. In contrast, the resistant strain created a fragile, hyper-connected network with higher mobility of mobile genetic elements (MGEs), which facilitated horizontal gene transfer.Host identification analysis confirmed this mechanism, showing the S treatment effectively reduced potential ARG hosts to only two genera (PseudomonasandMoheibacter), significantly fewer than the 11 and 7 hosts identified in the control and R treatments, respectively. Partial least squares path modeling (PLS-PM) revealed that the sensitive strain uniquely reduced the influence of MGEs while enhancing temperature's role in ARG reduction. The findings establish that employing antibiotic-sensitive functional strains is a reliable strategy to mitigate environmental antibiotic resistance risks.
Additional Links: PMID-41692309
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41692309,
year = {2026},
author = {Hao, X and Jiang, L and Chen, M and Liu, S and Zhu, L and Jiang, D and Bai, L},
title = {Antibiotic sensitivity as a key Determinant: B. Subtilis Reshapes the Microecology to mitigate antibiotic resistance genes during composting.},
journal = {Bioresource technology},
volume = {},
number = {},
pages = {134222},
doi = {10.1016/j.biortech.2026.134222},
pmid = {41692309},
issn = {1873-2976},
abstract = {This study aimed to investigate the role of inoculant antibiotic susceptibility in controlling antibiotic resistance genes (ARGs) during aerobic composting. A systematic comparison was conducted using Bacillus subtilis strains (sensitive, S; resistant, R) to assess ARG dynamics, microbial community evolution, and the underlying ecological mechanisms. Results demonstrated that the sensitive strain significantly enhanced composting efficiency, achieving a higher and longer-lasting secondary thermophilic phase (58.4°C for 4 days) and superior maturity indices compared to the resistant strain. Crucially, the R strain counteracted the ARG-removal effect of high temperatures, increasing total ARG abundance by 28.40% by day 6 and resulting in a final ARG burden 2.74 times higher than the S treatment.Microecological mechanism analysis revealed that the sensitive strain fostered a specialized, modular microbial network with reduced niche breadth, enhancing community stability and functioning as a genetic firewall to restrict ARG dissemination. In contrast, the resistant strain created a fragile, hyper-connected network with higher mobility of mobile genetic elements (MGEs), which facilitated horizontal gene transfer.Host identification analysis confirmed this mechanism, showing the S treatment effectively reduced potential ARG hosts to only two genera (PseudomonasandMoheibacter), significantly fewer than the 11 and 7 hosts identified in the control and R treatments, respectively. Partial least squares path modeling (PLS-PM) revealed that the sensitive strain uniquely reduced the influence of MGEs while enhancing temperature's role in ARG reduction. The findings establish that employing antibiotic-sensitive functional strains is a reliable strategy to mitigate environmental antibiotic resistance risks.},
}
RevDate: 2026-02-15
Unveiling the adaptive evolution of halotolerant aceticlastic methanogenesis: Multi-scale responses and energy partition.
Water research, 294:125552 pii:S0043-1354(26)00235-6 [Epub ahead of print].
The high concentration of salt ions in saline organic wastewater poses significant challenges for wastewater treatment technologies, particularly impacting the stability of anaerobic digesters. Aceticlastic methanogenesis is a crucial pathway for converting acetate into methane through methanoarchaea whose metabolism is adversely impacted by salt stress. To address this, long-term adaptive laboratory evolution (ALE) was conducted to cultivate halotolerant aceticlastic methanoarchaea, incorporating metagenomics, metatranscriptomic sequencing, metabolomics, and metabolic modeling to delineate genetic and metabolic responses. The evolved microbiome achieved a substantial increase in methanogenic activity at 5 % sodium chloride, reaching 82.25 % theoretical conversion of acetate to methane, significantly outperforming the original microbiome. This ALE process overcame the natural scarcity of aceticlastic methanogens in hypersaline environments. Key adaptation mechanisms were confirmed at the transcriptional level, primarily involving the upregulation of genes for inorganic ion transport, compatible solute uptake, and de novo biosynthesis. Horizontal gene transfer also contributed significantly through the transfer of osmoregulation genes, particularly those for compatible solute transport, suggesting an energy-efficient adaptation strategy of accumulating rather than synthesizing solutes. Metabolic flux analysis revealed that adjustments in energy distribution under salt stress are driven by the energetic cost of synthesizing compatible solutes, which highlights the importance of solute transporters for energy conservation. This study elucidates the complex interplay between metabolic reprogramming and gene transfer in enhancing microbial resilience under salt stress, thereby deepening our understanding of microbial adaptations in extreme environments and advancing biotechnological approaches for saline wastewater treatment.
Additional Links: PMID-41691814
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41691814,
year = {2026},
author = {Guo, H and Liu, Q and Han, H and Xu, W and Shi, W and Zhao, M and Xiao, X and Liu, J and Li, T},
title = {Unveiling the adaptive evolution of halotolerant aceticlastic methanogenesis: Multi-scale responses and energy partition.},
journal = {Water research},
volume = {294},
number = {},
pages = {125552},
doi = {10.1016/j.watres.2026.125552},
pmid = {41691814},
issn = {1879-2448},
abstract = {The high concentration of salt ions in saline organic wastewater poses significant challenges for wastewater treatment technologies, particularly impacting the stability of anaerobic digesters. Aceticlastic methanogenesis is a crucial pathway for converting acetate into methane through methanoarchaea whose metabolism is adversely impacted by salt stress. To address this, long-term adaptive laboratory evolution (ALE) was conducted to cultivate halotolerant aceticlastic methanoarchaea, incorporating metagenomics, metatranscriptomic sequencing, metabolomics, and metabolic modeling to delineate genetic and metabolic responses. The evolved microbiome achieved a substantial increase in methanogenic activity at 5 % sodium chloride, reaching 82.25 % theoretical conversion of acetate to methane, significantly outperforming the original microbiome. This ALE process overcame the natural scarcity of aceticlastic methanogens in hypersaline environments. Key adaptation mechanisms were confirmed at the transcriptional level, primarily involving the upregulation of genes for inorganic ion transport, compatible solute uptake, and de novo biosynthesis. Horizontal gene transfer also contributed significantly through the transfer of osmoregulation genes, particularly those for compatible solute transport, suggesting an energy-efficient adaptation strategy of accumulating rather than synthesizing solutes. Metabolic flux analysis revealed that adjustments in energy distribution under salt stress are driven by the energetic cost of synthesizing compatible solutes, which highlights the importance of solute transporters for energy conservation. This study elucidates the complex interplay between metabolic reprogramming and gene transfer in enhancing microbial resilience under salt stress, thereby deepening our understanding of microbial adaptations in extreme environments and advancing biotechnological approaches for saline wastewater treatment.},
}
RevDate: 2026-02-15
CmpDate: 2026-02-15
Formation of a novel multiresistance plasmid co-carrying tigecycline, carbapenem, and other resistance genes by recombination during conjugative transfer in Klebsiella pneumoniae.
International journal of antimicrobial agents, 67(2):107683.
OBJECTIVE: Klebsiella pneumoniae is a major global nosocomial pathogen, and strains acquiring extended-spectrum β-lactamase (ESBL) or carbapenemase resistance genes exhibit extensive clinical drug resistance, posing a serious public health threat. This study aimed to characterize the genetic features and transferability of resistance determinants in a clinically isolated multidrug-resistant K. pneumoniae strain.
METHODS: A multidrug-resistant K. pneumoniae strain was isolated from clinical samples. Whole-genome sequencing was performed to identify the resistance genes carried by the strain and the transposase sequences within the genetic environment of the target resistance genes. Conjugative transfer experiments were conducted to verify the transferability of the identified resistance genes and their genetic recombination characteristics.
RESULTS: The clinical isolate was confirmed to co-carry a tet(A) variant, tmexCD2-toprJ2, and blaNDM-1 resistance genes. Whole-genome sequencing revealed the presence of IS26, IS3000, and ∆tnpA transposase sequences in the genetic environment of tet(A)v and blaNDM-1 genes. Conjugative transfer experiments verified the transferability of the different resistance genes, and notably, recombination and co-transfer events of tet(A)v and blaNDM-1 genes were detected within the conjugative plasmid of the strain.
CONCLUSIONS: Transposases play a crucial role in the formation of complex multidrug-resistant K. pneumoniae strains. The findings of this study provide a novel perspective and critical evidence for elucidating the antimicrobial resistance mechanisms and dissemination pathways of multidrug-resistant K. pneumoniae.
Additional Links: PMID-41325814
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41325814,
year = {2026},
author = {Yu, R and Chen, Z and Schwarz, S and Yao, H and Li, C and Du, XD},
title = {Formation of a novel multiresistance plasmid co-carrying tigecycline, carbapenem, and other resistance genes by recombination during conjugative transfer in Klebsiella pneumoniae.},
journal = {International journal of antimicrobial agents},
volume = {67},
number = {2},
pages = {107683},
doi = {10.1016/j.ijantimicag.2025.107683},
pmid = {41325814},
issn = {1872-7913},
mesh = {*Klebsiella pneumoniae/genetics/drug effects/isolation & purification ; *Plasmids/genetics ; Humans ; *Drug Resistance, Multiple, Bacterial/genetics ; *Anti-Bacterial Agents/pharmacology ; Klebsiella Infections/microbiology ; Conjugation, Genetic ; *Tigecycline/pharmacology ; *Carbapenems/pharmacology ; Whole Genome Sequencing ; Microbial Sensitivity Tests ; *Recombination, Genetic ; beta-Lactamases/genetics ; Gene Transfer, Horizontal ; Bacterial Proteins/genetics ; },
abstract = {OBJECTIVE: Klebsiella pneumoniae is a major global nosocomial pathogen, and strains acquiring extended-spectrum β-lactamase (ESBL) or carbapenemase resistance genes exhibit extensive clinical drug resistance, posing a serious public health threat. This study aimed to characterize the genetic features and transferability of resistance determinants in a clinically isolated multidrug-resistant K. pneumoniae strain.
METHODS: A multidrug-resistant K. pneumoniae strain was isolated from clinical samples. Whole-genome sequencing was performed to identify the resistance genes carried by the strain and the transposase sequences within the genetic environment of the target resistance genes. Conjugative transfer experiments were conducted to verify the transferability of the identified resistance genes and their genetic recombination characteristics.
RESULTS: The clinical isolate was confirmed to co-carry a tet(A) variant, tmexCD2-toprJ2, and blaNDM-1 resistance genes. Whole-genome sequencing revealed the presence of IS26, IS3000, and ∆tnpA transposase sequences in the genetic environment of tet(A)v and blaNDM-1 genes. Conjugative transfer experiments verified the transferability of the different resistance genes, and notably, recombination and co-transfer events of tet(A)v and blaNDM-1 genes were detected within the conjugative plasmid of the strain.
CONCLUSIONS: Transposases play a crucial role in the formation of complex multidrug-resistant K. pneumoniae strains. The findings of this study provide a novel perspective and critical evidence for elucidating the antimicrobial resistance mechanisms and dissemination pathways of multidrug-resistant K. pneumoniae.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Klebsiella pneumoniae/genetics/drug effects/isolation & purification
*Plasmids/genetics
Humans
*Drug Resistance, Multiple, Bacterial/genetics
*Anti-Bacterial Agents/pharmacology
Klebsiella Infections/microbiology
Conjugation, Genetic
*Tigecycline/pharmacology
*Carbapenems/pharmacology
Whole Genome Sequencing
Microbial Sensitivity Tests
*Recombination, Genetic
beta-Lactamases/genetics
Gene Transfer, Horizontal
Bacterial Proteins/genetics
RevDate: 2026-02-14
Ancestral neuronal receptors are bacterial accessory toxins.
Nature communications pii:10.1038/s41467-026-69246-x [Epub ahead of print].
Horizontal gene transfer events were crucial in the emergence of multicellular life. A striking example is the acquisition of Teneurins, putative surface-exposed toxins in bacteria that function as cell adhesion receptors in metazoan neuronal development. Here, we demonstrate the evolutionary relationships between metazoan and bacterial Teneurins. We use cryogenic electron microscopy and bioinformatic analysis to show that bacterial Teneurins harbour a toxic protein in a proteinaceous shell. They are rare but widely distributed across bacterial taxa and are predominantly seen in species with complex social behaviours, suggesting roles in cell-to-cell interaction. This work confirms that metazoan Teneurins are repurposed bacterial toxins that have evolved to be essential mediators of intercellular communication in all advanced nervous systems. Their acquisition was a key event in the evolution of metazoans.
Additional Links: PMID-41690916
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41690916,
year = {2026},
author = {Raoelijaona, F and Szczepaniak, J and Schahl, A and Bray, JE and Zhou, JC and Baker, L and El Omari, K and Lowe, E and Low, YS and Rodriguez, CM and Landsberg, MJ and Lott, JS and Kleanthous, C and Chavent, M and Maiden, MC and Seiradake, E},
title = {Ancestral neuronal receptors are bacterial accessory toxins.},
journal = {Nature communications},
volume = {},
number = {},
pages = {},
doi = {10.1038/s41467-026-69246-x},
pmid = {41690916},
issn = {2041-1723},
support = {202827/Z/16/Z//Wellcome Trust (Wellcome)/ ; 226647/Z/22/Z//Wellcome Trust (Wellcome)/ ; 218205/Z/19/Z//Wellcome Trust (Wellcome)/ ; EMBO Young Investigator Programme//European Molecular Biology Organization (EMBO)/ ; },
abstract = {Horizontal gene transfer events were crucial in the emergence of multicellular life. A striking example is the acquisition of Teneurins, putative surface-exposed toxins in bacteria that function as cell adhesion receptors in metazoan neuronal development. Here, we demonstrate the evolutionary relationships between metazoan and bacterial Teneurins. We use cryogenic electron microscopy and bioinformatic analysis to show that bacterial Teneurins harbour a toxic protein in a proteinaceous shell. They are rare but widely distributed across bacterial taxa and are predominantly seen in species with complex social behaviours, suggesting roles in cell-to-cell interaction. This work confirms that metazoan Teneurins are repurposed bacterial toxins that have evolved to be essential mediators of intercellular communication in all advanced nervous systems. Their acquisition was a key event in the evolution of metazoans.},
}
RevDate: 2026-02-14
Copper resistance and genetic determinants in Chilean strains of Clavibacter michiganensis the causal agent of bacterial canker of tomato.
Pest management science [Epub ahead of print].
BACKGROUND: The control of Clavibacter michiganensis, the causal agent of bacterial canker in tomato, remains a significant challenge for crop cultivation. While copper-based products are the most commonly used bactericides, their efficacy against this pathogen is often inefficient. Therefore, the objective of this study was to determine the copper susceptibility of five Chilean Clavibacter michiganensis strains and to characterize their associated copper resistance gene repertoire.
RESULTS: Chilean strains VQ28, VQ143, and VL527 showed moderate copper resistance, being able to grow at a concentration ≤ 0.32 mm of copper in CYEG medium. In contrast, strains OP3 and MSF322 showed higher copper resistance, growing at a copper-concentration ≤ 0.4 mm. The search for genes associated with copper resistance revealed the presence of the copA, copC, copD, copZ, ycnI and ycnJ genes and the csoR1 regulator gene in the chromosomes of all the strains analyzed. The presence and location of the csoR2 and csoR3 regulators genes varied among the strains. Strains MSF322 and OP3, shown to be more tolerant to copper, possess a copB gene located in a plasmid which was not found in other Chilean strains. Notably, strain OP3, isolated in 2015 - years after the other strains - harbors copper resistance genes on plasmids highly similar to those in other Chilean strains, suggesting recent horizontal gene transfer.
CONCLUSION: Chilean strains of Clavibacter michiganensis exhibit moderate tolerance to copper, and the acquisition of new genes through horizontal gene transfer could play a crucial role in Clavibacter michiganensis copper resistance. © 2026 Society of Chemical Industry.
Additional Links: PMID-41689363
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41689363,
year = {2026},
author = {Valenzuela, M and Vásconez, IN and Méndez, V and Fuentes, B and Altimira, F and Valdés, F and Montenegro, I and Besoain, X and Seeger, M},
title = {Copper resistance and genetic determinants in Chilean strains of Clavibacter michiganensis the causal agent of bacterial canker of tomato.},
journal = {Pest management science},
volume = {},
number = {},
pages = {},
doi = {10.1002/ps.70665},
pmid = {41689363},
issn = {1526-4998},
support = {//Universidad Técnica Federico Santa María/ ; //Agencia Nacional de Investigación y Desarrollo/ ; //GORE & CORE Region de O'Higgins/ ; },
abstract = {BACKGROUND: The control of Clavibacter michiganensis, the causal agent of bacterial canker in tomato, remains a significant challenge for crop cultivation. While copper-based products are the most commonly used bactericides, their efficacy against this pathogen is often inefficient. Therefore, the objective of this study was to determine the copper susceptibility of five Chilean Clavibacter michiganensis strains and to characterize their associated copper resistance gene repertoire.
RESULTS: Chilean strains VQ28, VQ143, and VL527 showed moderate copper resistance, being able to grow at a concentration ≤ 0.32 mm of copper in CYEG medium. In contrast, strains OP3 and MSF322 showed higher copper resistance, growing at a copper-concentration ≤ 0.4 mm. The search for genes associated with copper resistance revealed the presence of the copA, copC, copD, copZ, ycnI and ycnJ genes and the csoR1 regulator gene in the chromosomes of all the strains analyzed. The presence and location of the csoR2 and csoR3 regulators genes varied among the strains. Strains MSF322 and OP3, shown to be more tolerant to copper, possess a copB gene located in a plasmid which was not found in other Chilean strains. Notably, strain OP3, isolated in 2015 - years after the other strains - harbors copper resistance genes on plasmids highly similar to those in other Chilean strains, suggesting recent horizontal gene transfer.
CONCLUSION: Chilean strains of Clavibacter michiganensis exhibit moderate tolerance to copper, and the acquisition of new genes through horizontal gene transfer could play a crucial role in Clavibacter michiganensis copper resistance. © 2026 Society of Chemical Industry.},
}
RevDate: 2026-02-13
Bacterial defense systems and host ecology drive the evolution of intra-species lineages.
Cell reports, 45(2):116957 pii:S2211-1247(26)00035-5 [Epub ahead of print].
Horizontal gene transfer (HGT) is a major driver of diversity in bacterial populations. However, our understanding of its impact on the evolution of intra-species lineages is limited. The multi-host bacterial pathogen Staphylococcus aureus is differentiated into genetic lineages known as clonal complexes (CC) with variable host and disease tropisms. Here, we demonstrate that CCs exhibit extensive variation in pangenome size, structure, and gene flow, influenced by both genetic and ecological barriers to HGT. Examination of pangenome openness for each CC revealed remarkable variation that correlated strongly with host-species promiscuity. Notably, CCs were defined by horizontally acquired defense systems, and genetic subpopulations have diverged by changes to their type I restriction-modification (R-M) system repertoire, suggesting a role in lineage emergence. Overall, our data indicate a key role of HGT of defense systems in promoting the differentiation of S. aureus into lineages, with host ecology as a major driver of accessory genome variation.
Additional Links: PMID-41686637
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41686637,
year = {2026},
author = {Gorzynski, J and Harling-Lee, JD and Figueroa, W and Alves, J and Yebra, G and Freeman, T and Penadés, JR and Fitzgerald, JR},
title = {Bacterial defense systems and host ecology drive the evolution of intra-species lineages.},
journal = {Cell reports},
volume = {45},
number = {2},
pages = {116957},
doi = {10.1016/j.celrep.2026.116957},
pmid = {41686637},
issn = {2211-1247},
abstract = {Horizontal gene transfer (HGT) is a major driver of diversity in bacterial populations. However, our understanding of its impact on the evolution of intra-species lineages is limited. The multi-host bacterial pathogen Staphylococcus aureus is differentiated into genetic lineages known as clonal complexes (CC) with variable host and disease tropisms. Here, we demonstrate that CCs exhibit extensive variation in pangenome size, structure, and gene flow, influenced by both genetic and ecological barriers to HGT. Examination of pangenome openness for each CC revealed remarkable variation that correlated strongly with host-species promiscuity. Notably, CCs were defined by horizontally acquired defense systems, and genetic subpopulations have diverged by changes to their type I restriction-modification (R-M) system repertoire, suggesting a role in lineage emergence. Overall, our data indicate a key role of HGT of defense systems in promoting the differentiation of S. aureus into lineages, with host ecology as a major driver of accessory genome variation.},
}
RevDate: 2026-02-13
CmpDate: 2026-02-13
Horizontal gene transfer and gene loss drove the divergent evolution of host dependency in Micrarchaeota.
National science review, 13(4):nwaf542.
The DPANN superphylum is a deep-branching radiation of archaea with small cell and genome sizes. Most DPANN lineages are predicted or validated to be host-dependent. However, certain lineages have substantial biosynthetic capacities and are potentially less dependent on hosts, or even free-living. Here, we reconstructed 163 Micrarchaeota genomes, comprising 48 assigned to previously undescribed orders and 115 affiliated with known orders. Investigation of their genetic repertoire revealed substantial metabolic capacity in Norongarragalinales-, Anstonellales- and the newly proposed Wunengiarchaeales-associated lineages, including complete or near-complete glycolysis and de novo biosynthetic pathways for nucleotides, amino acids, cofactors and cell envelopes. We classified genes related to the central metabolism but which are uncommon in DPANN archaea as putative free-living associated genes (pFLAGs). The extensive presence of pFLAGs in Norongarragalinales suggests a potential host-independent lifestyle. Reconstruction of evolutionary history revealed that these pFLAGs were not ancestral within the DPANN superphylum. Instead, we suggest that less-host-dependent organisms evolved from symbionts through the gradual acquisition of pFLAGs through horizontal gene transfer, whereas other Micrarchaeota lineages with streamlined genomes experienced reductive evolution due to thermal adaptation. Our analyses demonstrate that host dependency is not always an evolutionary dead end, but can be reversed through the acquisition of new metabolic capabilities by horizontal transfer.
Additional Links: PMID-41684701
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41684701,
year = {2026},
author = {Rao, YZ and Li, YX and Li, ZW and Qu, YN and Hedlund, BP and Williams, TA and Qi, YL and Xie, QJ and Yang, HL and Zhang, YQ and Jiang, HC and Palmer, M and Shi, M and Shu, WS and Hua, ZS and Li, WJ},
title = {Horizontal gene transfer and gene loss drove the divergent evolution of host dependency in Micrarchaeota.},
journal = {National science review},
volume = {13},
number = {4},
pages = {nwaf542},
pmid = {41684701},
issn = {2053-714X},
abstract = {The DPANN superphylum is a deep-branching radiation of archaea with small cell and genome sizes. Most DPANN lineages are predicted or validated to be host-dependent. However, certain lineages have substantial biosynthetic capacities and are potentially less dependent on hosts, or even free-living. Here, we reconstructed 163 Micrarchaeota genomes, comprising 48 assigned to previously undescribed orders and 115 affiliated with known orders. Investigation of their genetic repertoire revealed substantial metabolic capacity in Norongarragalinales-, Anstonellales- and the newly proposed Wunengiarchaeales-associated lineages, including complete or near-complete glycolysis and de novo biosynthetic pathways for nucleotides, amino acids, cofactors and cell envelopes. We classified genes related to the central metabolism but which are uncommon in DPANN archaea as putative free-living associated genes (pFLAGs). The extensive presence of pFLAGs in Norongarragalinales suggests a potential host-independent lifestyle. Reconstruction of evolutionary history revealed that these pFLAGs were not ancestral within the DPANN superphylum. Instead, we suggest that less-host-dependent organisms evolved from symbionts through the gradual acquisition of pFLAGs through horizontal gene transfer, whereas other Micrarchaeota lineages with streamlined genomes experienced reductive evolution due to thermal adaptation. Our analyses demonstrate that host dependency is not always an evolutionary dead end, but can be reversed through the acquisition of new metabolic capabilities by horizontal transfer.},
}
RevDate: 2026-02-13
CmpDate: 2026-02-13
Microbial community structure and functional potential in a long-term uranium-nickel contaminated ecosystem.
Frontiers in microbiology, 17:1741152.
This study examined the microbial community structure, functional potential, and resistance determinants in uranium (U)- and nickel (Ni)-contaminated soils from the Savannah River Site (SRS), a former nuclear materials production and waste collection facility operated by the U. S. Department of Energy (DOE). Soil cores were collected from the Steed Pond area, where long-term discharge of acidic wastewater resulted in spatially variable contamination levels. Concentrations of U and Ni in the collected samples ranged from 0.22-10.44 g kg[-1] and 0.79-2.28 g kg[-1], respectively. Shotgun metagenomic and high-throughput quantitative PCR (HT-qPCR) analyses revealed bacterial communities dominated by Pseudomonadota, Actinomycetota, and Acidobacteriota, with enrichment of taxa affiliated with genera known to include diazotrophic members (e.g., Bradyrhizobium and Burkholderia), alongside increased abundance of nitrogen fixation-related functional genes. Carbon and nitrogen cycle genes were generally well represented across samples, with selective shifts observed in acetate assimilation genes (acsA/acsE) and comparatively low abundance of hydrazine oxidoreductase (hzo), indicating pathway-specific variation rather than broad metabolic suppression. A total of 117 resistance-associated genes were identified, comprising 93 antibiotic-resistance genes (ARGs), 3 metal-resistance genes (MRGs), and 21 mobile genetic elements (MGEs). Strong positive correlations among ARGs, MRGs, and MGEs indicate co-selection and horizontal gene transfer, forming a genetically mobile resistome. Collectively, these findings demonstrate that long-term U-Ni contamination selects for metabolically versatile, diazotroph-enriched, and genetically mobile microbiomes. Such communities exhibit both resistance proliferation and bioremediation potential, providing key insights into microbial adaptation and ecosystem recovery in legacy nuclear-contaminated soils.
Additional Links: PMID-41684676
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41684676,
year = {2026},
author = {Chukwujindu, C and Kolton, M and Fasakin, O and Pathak, A and Seaman, J and Chauhan, A},
title = {Microbial community structure and functional potential in a long-term uranium-nickel contaminated ecosystem.},
journal = {Frontiers in microbiology},
volume = {17},
number = {},
pages = {1741152},
pmid = {41684676},
issn = {1664-302X},
abstract = {This study examined the microbial community structure, functional potential, and resistance determinants in uranium (U)- and nickel (Ni)-contaminated soils from the Savannah River Site (SRS), a former nuclear materials production and waste collection facility operated by the U. S. Department of Energy (DOE). Soil cores were collected from the Steed Pond area, where long-term discharge of acidic wastewater resulted in spatially variable contamination levels. Concentrations of U and Ni in the collected samples ranged from 0.22-10.44 g kg[-1] and 0.79-2.28 g kg[-1], respectively. Shotgun metagenomic and high-throughput quantitative PCR (HT-qPCR) analyses revealed bacterial communities dominated by Pseudomonadota, Actinomycetota, and Acidobacteriota, with enrichment of taxa affiliated with genera known to include diazotrophic members (e.g., Bradyrhizobium and Burkholderia), alongside increased abundance of nitrogen fixation-related functional genes. Carbon and nitrogen cycle genes were generally well represented across samples, with selective shifts observed in acetate assimilation genes (acsA/acsE) and comparatively low abundance of hydrazine oxidoreductase (hzo), indicating pathway-specific variation rather than broad metabolic suppression. A total of 117 resistance-associated genes were identified, comprising 93 antibiotic-resistance genes (ARGs), 3 metal-resistance genes (MRGs), and 21 mobile genetic elements (MGEs). Strong positive correlations among ARGs, MRGs, and MGEs indicate co-selection and horizontal gene transfer, forming a genetically mobile resistome. Collectively, these findings demonstrate that long-term U-Ni contamination selects for metabolically versatile, diazotroph-enriched, and genetically mobile microbiomes. Such communities exhibit both resistance proliferation and bioremediation potential, providing key insights into microbial adaptation and ecosystem recovery in legacy nuclear-contaminated soils.},
}
RevDate: 2026-02-13
CmpDate: 2026-02-13
Integrative and conjugative elements in Mycoplasmopsis bovis from Western Canadian feedlot cattle: characterization and conjugative transfer.
Frontiers in veterinary science, 13:1719776.
INTRODUCTION: Bovine respiratory disease (BRD) is the most significant disease affecting North American feedlot cattle. It is a multifactorial disease influenced by bacterial and viral pathogens, as well as management and environmental factors. Mycoplasmopsis bovis is among the most pathogenic bovine mycoplasmas and is associated with chronic BRD that often fails to respond to antimicrobial therapy. Integrative and conjugative elements (ICE) facilitate horizontal gene transfer among mycoplasmas and may contribute to the spread of antimicrobial resistance in M. bovis.
METHODS: We identified mycoplasma ICEs (MICE) in the genomes of sequenced M. bovis isolates from western Canadian feedlot cattle (n = 124) and in vitro mating experiments to assess conjugation.
RESULTS AND DISCUSSION: Of these isolates, 33.1% harbored the array of MICE genes required for conjugation. M. bovis isolates conjugated at frequencies of 10-7-10-8 when cultured in SP4 broth under orbital agitation. Since MICE circularization is the initial step in conjugation, the presence of circular MICE (cMICE) was used as a proxy for conjugation capability (n = 451). Interestingly, 25.7% of the isolates were cMICE-positive, with a higher prevalence observed in M. bovis isolated from dairy as compared to beef feedlot cattle. Additionally, calves classified as high-risk for BRD were more likely to harbor cMICE-positive M. bovis in both cattle types. Backgrounded dairy cattle had a higher likelihood of carrying cMICE-positive M. bovis than those originating from ranches. These findings lay the groundwork for assessing cattle source as a determinant of cMICE-positive M. bovis and for developing targeted strategies to mitigate antimicrobial resistance.
Additional Links: PMID-41684385
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41684385,
year = {2026},
author = {Andres-Lasheras, S and Zaheer, R and Ortega-Polo, R and Schwinghamer, T and Abeysekara, S and Zovoilis, A and Zaidi, SE and Jelinski, M and McAllister, TA},
title = {Integrative and conjugative elements in Mycoplasmopsis bovis from Western Canadian feedlot cattle: characterization and conjugative transfer.},
journal = {Frontiers in veterinary science},
volume = {13},
number = {},
pages = {1719776},
pmid = {41684385},
issn = {2297-1769},
abstract = {INTRODUCTION: Bovine respiratory disease (BRD) is the most significant disease affecting North American feedlot cattle. It is a multifactorial disease influenced by bacterial and viral pathogens, as well as management and environmental factors. Mycoplasmopsis bovis is among the most pathogenic bovine mycoplasmas and is associated with chronic BRD that often fails to respond to antimicrobial therapy. Integrative and conjugative elements (ICE) facilitate horizontal gene transfer among mycoplasmas and may contribute to the spread of antimicrobial resistance in M. bovis.
METHODS: We identified mycoplasma ICEs (MICE) in the genomes of sequenced M. bovis isolates from western Canadian feedlot cattle (n = 124) and in vitro mating experiments to assess conjugation.
RESULTS AND DISCUSSION: Of these isolates, 33.1% harbored the array of MICE genes required for conjugation. M. bovis isolates conjugated at frequencies of 10-7-10-8 when cultured in SP4 broth under orbital agitation. Since MICE circularization is the initial step in conjugation, the presence of circular MICE (cMICE) was used as a proxy for conjugation capability (n = 451). Interestingly, 25.7% of the isolates were cMICE-positive, with a higher prevalence observed in M. bovis isolated from dairy as compared to beef feedlot cattle. Additionally, calves classified as high-risk for BRD were more likely to harbor cMICE-positive M. bovis in both cattle types. Backgrounded dairy cattle had a higher likelihood of carrying cMICE-positive M. bovis than those originating from ranches. These findings lay the groundwork for assessing cattle source as a determinant of cMICE-positive M. bovis and for developing targeted strategies to mitigate antimicrobial resistance.},
}
RevDate: 2026-02-13
CmpDate: 2026-02-13
Unveiling hidden risks of chiral fungicide benzovindiflupyr: Stereoselectivity in soil antibiotic resistance gene transmission.
Journal of hazardous materials, 503:141088.
Antibiotic resistance gene (ARG) dissemination is closely associated with modern agricultural practices. However, the stereoselective effects of widely applied chiral pesticides on resistance evolution remain insufficiently investigated. This study systematically explored the differential effects of benzovindiflupyr enantiomers on transmission of ARGs through long-term soil incubation experiments combined with metagenomic and in vitro studies. Results demonstrated that 1S,4R-enantiomer exhibited significantly longer half-life than 1 R,4S-enantiomer. 1 R,4S-enantiomer induced extreme enrichment of a few ARGs. 1S,4R-enantiomer persistently increased abundance of multiple ARGs. Compared with 1 R,4S-enantiomer, 1S,4R-enantiomer more consistently enhanced abundance of mobile genetic elements (MGEs) related to conjugative transfer. Moreover, 1 R,4S-enantiomer primarily enriched specific genera within Pseudomonadota. 1S,4R-enantiomer simultaneously promoted abundance of multiple genera across both Pseudomonadota and Bacteroidota, driving cross-phylum genera to correlate with shared ARGs. Genomic analysis confirmed that Pseudomonadota under 1S,4R-enantiomer treatment carried more ARGs and MGEs. In vitro transformation experiments ultimately validated that 1S,4R-enantiomer significantly enhanced transformation efficiency across multiple ARGs consistently, substantially exceeding 1 R,4S-enantiomer effects. Overall, 1S,4R-enantiomer poses more significant risks for horizontal transfer of ARGs. This study elucidates enantioselective effects of chiral pesticides on transmission of ARGs, providing a foundation for improving chiral agrochemical risk assessment.
Additional Links: PMID-41538947
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41538947,
year = {2026},
author = {Zhang, X and Feng, Y and Jiang, X and Sun, W and Zhang, C and Han, J and Hou, Y and You, X and Zhang, H and Wang, X and Wu, X and Wang, J},
title = {Unveiling hidden risks of chiral fungicide benzovindiflupyr: Stereoselectivity in soil antibiotic resistance gene transmission.},
journal = {Journal of hazardous materials},
volume = {503},
number = {},
pages = {141088},
doi = {10.1016/j.jhazmat.2026.141088},
pmid = {41538947},
issn = {1873-3336},
mesh = {*Soil Microbiology ; Stereoisomerism ; *Fungicides, Industrial/chemistry/pharmacology/toxicity ; *Soil Pollutants/chemistry/toxicity ; *Drug Resistance, Microbial/genetics ; Genes, Bacterial ; Gene Transfer, Horizontal ; Soil/chemistry ; },
abstract = {Antibiotic resistance gene (ARG) dissemination is closely associated with modern agricultural practices. However, the stereoselective effects of widely applied chiral pesticides on resistance evolution remain insufficiently investigated. This study systematically explored the differential effects of benzovindiflupyr enantiomers on transmission of ARGs through long-term soil incubation experiments combined with metagenomic and in vitro studies. Results demonstrated that 1S,4R-enantiomer exhibited significantly longer half-life than 1 R,4S-enantiomer. 1 R,4S-enantiomer induced extreme enrichment of a few ARGs. 1S,4R-enantiomer persistently increased abundance of multiple ARGs. Compared with 1 R,4S-enantiomer, 1S,4R-enantiomer more consistently enhanced abundance of mobile genetic elements (MGEs) related to conjugative transfer. Moreover, 1 R,4S-enantiomer primarily enriched specific genera within Pseudomonadota. 1S,4R-enantiomer simultaneously promoted abundance of multiple genera across both Pseudomonadota and Bacteroidota, driving cross-phylum genera to correlate with shared ARGs. Genomic analysis confirmed that Pseudomonadota under 1S,4R-enantiomer treatment carried more ARGs and MGEs. In vitro transformation experiments ultimately validated that 1S,4R-enantiomer significantly enhanced transformation efficiency across multiple ARGs consistently, substantially exceeding 1 R,4S-enantiomer effects. Overall, 1S,4R-enantiomer poses more significant risks for horizontal transfer of ARGs. This study elucidates enantioselective effects of chiral pesticides on transmission of ARGs, providing a foundation for improving chiral agrochemical risk assessment.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Soil Microbiology
Stereoisomerism
*Fungicides, Industrial/chemistry/pharmacology/toxicity
*Soil Pollutants/chemistry/toxicity
*Drug Resistance, Microbial/genetics
Genes, Bacterial
Gene Transfer, Horizontal
Soil/chemistry
RevDate: 2026-02-12
Tet(X4)-Producing Escherichia coli Isolates in Taiwan.
Journal of global antimicrobial resistance pii:S2213-7165(26)00015-9 [Epub ahead of print].
BACKGROUND: Plasmid-mediated tet(X4), linked to high-level tigecycline resistance, was first identified in China with Escherichia coli (E. coli) as a major reservoir. No confirmed cases had been reported in Taiwan.
METHODS: We examined 81 tigecycline-resistant E. coli isolates (MIC ≥ 4 mg/L) collected in Taiwan from 2015-2022, including 71.6% carbapenem-resistant and 28.4% carbapenem-susceptible strains. Thirty-six underwent whole-genome sequencing to investigate resistance mechanisms.
RESULTS: Two isolates (2.5%) carried tet(X4) on novel plasmids (pEC1360-1 and pEC1638-1). Both plasmids contained the ISVsa3-estT-tet(X4)-ISVsa3 (IETI) element, a mobile unit capable of transposon-mediated transfer without a fixed integration hotspot. The tet(X4)-positive strains showed distinct evolutionary divergence from the first reported Chinese strain (LHM10-1). Tet(X4) was located on different Inc-type plasmids, including a 66.8 kb IncR and a 159.3 kb IncR/IncFIB(K)/IncFIA(HI1) plasmid, across various sequence types. No tet(X4) was detected in carbapenem-resistant isolates. Other resistance genes, such as cmlA1 and floR, were more prevalent in carbapenem-susceptible isolates (66.7% vs. 25.9%, P = 0.077).
CONCLUSION: This study reports the first tet(X4)-positive E. coli isolates in Taiwan, both from carbapenem-susceptible strains. The presence of novel mobile plasmids underscores the potential for horizontal gene transfer. Continuous surveillance of tet(X) and other last-line antibiotic resistance mechanisms is essential to mitigate the risk of further spread.
Additional Links: PMID-41679514
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41679514,
year = {2026},
author = {Huang, WC and Huang, YT and Ko, WC and Shih, WA and Teng, CH and Wang, JL},
title = {Tet(X4)-Producing Escherichia coli Isolates in Taiwan.},
journal = {Journal of global antimicrobial resistance},
volume = {},
number = {},
pages = {},
doi = {10.1016/j.jgar.2026.01.014},
pmid = {41679514},
issn = {2213-7173},
abstract = {BACKGROUND: Plasmid-mediated tet(X4), linked to high-level tigecycline resistance, was first identified in China with Escherichia coli (E. coli) as a major reservoir. No confirmed cases had been reported in Taiwan.
METHODS: We examined 81 tigecycline-resistant E. coli isolates (MIC ≥ 4 mg/L) collected in Taiwan from 2015-2022, including 71.6% carbapenem-resistant and 28.4% carbapenem-susceptible strains. Thirty-six underwent whole-genome sequencing to investigate resistance mechanisms.
RESULTS: Two isolates (2.5%) carried tet(X4) on novel plasmids (pEC1360-1 and pEC1638-1). Both plasmids contained the ISVsa3-estT-tet(X4)-ISVsa3 (IETI) element, a mobile unit capable of transposon-mediated transfer without a fixed integration hotspot. The tet(X4)-positive strains showed distinct evolutionary divergence from the first reported Chinese strain (LHM10-1). Tet(X4) was located on different Inc-type plasmids, including a 66.8 kb IncR and a 159.3 kb IncR/IncFIB(K)/IncFIA(HI1) plasmid, across various sequence types. No tet(X4) was detected in carbapenem-resistant isolates. Other resistance genes, such as cmlA1 and floR, were more prevalent in carbapenem-susceptible isolates (66.7% vs. 25.9%, P = 0.077).
CONCLUSION: This study reports the first tet(X4)-positive E. coli isolates in Taiwan, both from carbapenem-susceptible strains. The presence of novel mobile plasmids underscores the potential for horizontal gene transfer. Continuous surveillance of tet(X) and other last-line antibiotic resistance mechanisms is essential to mitigate the risk of further spread.},
}
RevDate: 2026-02-12
Cladophora drives the evolution of its epiphytic communities and antibiotic resistome in the littoral zone of Qinghai Lake.
Water research, 294:125530 pii:S0043-1354(26)00212-5 [Epub ahead of print].
Cladophora blooms, exacerbated by climate change and littoral eutrophication, pose a significant ecological threat. Of particular concern is their potential to disrupt phytoplankton and bacterial assemblages, triggering a cascade of effects that may include shifts in nutrient cycling and the dissemination of resistomes. However, the mechanistic links between Cladophora's life-stage-dependent dissolved organic matter (DOM) release, its role in restructuring epiphytic communities, and its promotion of resistome dissemination in natural, oligotrophic lakes remain poorly understood. To address this, this study integrates field and laboratory investigations of Cladophora qinghaiensis sp. nov.. The algal phycosphere functions as a dynamic "gene incubator", driven by chemical shifts in algal‑derived DOM. During decay under low‑oxygen conditions, DOM composition transitions from tyrosine‑like proteins to recalcitrant fulvic‑acid‑like compounds, selectively enriching competitive, intrinsically resistant taxa such as Halomonas and Phacus. Microbes such as Acinetobacter drive nutrient cycling (e.g., nitrogen metabolism) and serve as hotspots for resistomes within the phycosphere. Contrary to the expectation that high cell density favors horizontal gene transfer (HGT), genomic analyses show that vertical gene transfer (VGT) dominates antibiotic resistance gene (ARG) proliferation in this niche, a pattern explained by strong DOM‑mediated host selection and subsequent propagation. In contrast, the resistome in the surrounding water is more diverse and primarily shaped by HGT via mobile genetic elements. These results establish a mechanistic link between life‑stage‑specific algal DOM components, selective epiphytic communities enrichment, and divergent pathways of resistome evolution, positioning the phycosphere as a key source of ARGs that amplifies ecological risk in nearshore environments.
Additional Links: PMID-41679041
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41679041,
year = {2026},
author = {Jia, J and Ao, H and Xiong, X and Wang, S and Xi, X and Chen, K and Wu, C},
title = {Cladophora drives the evolution of its epiphytic communities and antibiotic resistome in the littoral zone of Qinghai Lake.},
journal = {Water research},
volume = {294},
number = {},
pages = {125530},
doi = {10.1016/j.watres.2026.125530},
pmid = {41679041},
issn = {1879-2448},
abstract = {Cladophora blooms, exacerbated by climate change and littoral eutrophication, pose a significant ecological threat. Of particular concern is their potential to disrupt phytoplankton and bacterial assemblages, triggering a cascade of effects that may include shifts in nutrient cycling and the dissemination of resistomes. However, the mechanistic links between Cladophora's life-stage-dependent dissolved organic matter (DOM) release, its role in restructuring epiphytic communities, and its promotion of resistome dissemination in natural, oligotrophic lakes remain poorly understood. To address this, this study integrates field and laboratory investigations of Cladophora qinghaiensis sp. nov.. The algal phycosphere functions as a dynamic "gene incubator", driven by chemical shifts in algal‑derived DOM. During decay under low‑oxygen conditions, DOM composition transitions from tyrosine‑like proteins to recalcitrant fulvic‑acid‑like compounds, selectively enriching competitive, intrinsically resistant taxa such as Halomonas and Phacus. Microbes such as Acinetobacter drive nutrient cycling (e.g., nitrogen metabolism) and serve as hotspots for resistomes within the phycosphere. Contrary to the expectation that high cell density favors horizontal gene transfer (HGT), genomic analyses show that vertical gene transfer (VGT) dominates antibiotic resistance gene (ARG) proliferation in this niche, a pattern explained by strong DOM‑mediated host selection and subsequent propagation. In contrast, the resistome in the surrounding water is more diverse and primarily shaped by HGT via mobile genetic elements. These results establish a mechanistic link between life‑stage‑specific algal DOM components, selective epiphytic communities enrichment, and divergent pathways of resistome evolution, positioning the phycosphere as a key source of ARGs that amplifies ecological risk in nearshore environments.},
}
RevDate: 2026-02-12
CmpDate: 2026-02-12
mcr gene family evolution and structural mechanisms of colistin resistance: from mcr-1 to emerging variants.
Archives of microbiology, 208(4):186.
The mcr gene family, responsible for plasmid-mediated resistance to colistin, poses a growing threat to public health by reducing the efficacy of colistin, a critical last-resort antibiotic for multidrug-resistant Gram-negative bacteria. The mcr-1 gene, discovered in 2015, marked a significant shift in understanding colistin resistance, and subsequent mcr variants (mcr-2 to mcr-10) have emerged globally. These genes alter lipid A in bacterial cell membranes, decreasing colistin's binding and efficacy. The mcr genes are typically located on mobile plasmids, facilitating horizontal gene transfer across bacterial species. Our review examines the evolution, genetic mechanisms, and structural characteristics of the mcr gene family, discussing their spread across human, animal, and environmental contexts. In this review, we highlight the clinical implications of mcr-mediated resistance, noting the co-occurrence of mcr with other antimicrobial resistance determinants, which complicates treatment options. Additionally, it explores detection methods, global epidemiology, and potential strategies to combat mcr resistance, including the development of inhibitors and CRISPR-based gene editing. Our review concludes that combating mcr-mediated colistin resistance requires global surveillance, coordination across sectors, and continued research to stop its spread and impact.
Additional Links: PMID-41677891
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41677891,
year = {2026},
author = {Mehmood, MS and Marakkalage, UKRK and Arif, T and Javaid, F and Abid, MA and Shahid, M and Parvez, A and Saddique, MN and Ali, S},
title = {mcr gene family evolution and structural mechanisms of colistin resistance: from mcr-1 to emerging variants.},
journal = {Archives of microbiology},
volume = {208},
number = {4},
pages = {186},
pmid = {41677891},
issn = {1432-072X},
mesh = {*Colistin/pharmacology ; *Anti-Bacterial Agents/pharmacology ; Humans ; *Drug Resistance, Bacterial/genetics ; Evolution, Molecular ; Animals ; *Gram-Negative Bacteria/genetics/drug effects ; *Bacterial Proteins/genetics/metabolism ; Gene Transfer, Horizontal ; Plasmids/genetics ; Multigene Family ; Escherichia coli Proteins ; },
abstract = {The mcr gene family, responsible for plasmid-mediated resistance to colistin, poses a growing threat to public health by reducing the efficacy of colistin, a critical last-resort antibiotic for multidrug-resistant Gram-negative bacteria. The mcr-1 gene, discovered in 2015, marked a significant shift in understanding colistin resistance, and subsequent mcr variants (mcr-2 to mcr-10) have emerged globally. These genes alter lipid A in bacterial cell membranes, decreasing colistin's binding and efficacy. The mcr genes are typically located on mobile plasmids, facilitating horizontal gene transfer across bacterial species. Our review examines the evolution, genetic mechanisms, and structural characteristics of the mcr gene family, discussing their spread across human, animal, and environmental contexts. In this review, we highlight the clinical implications of mcr-mediated resistance, noting the co-occurrence of mcr with other antimicrobial resistance determinants, which complicates treatment options. Additionally, it explores detection methods, global epidemiology, and potential strategies to combat mcr resistance, including the development of inhibitors and CRISPR-based gene editing. Our review concludes that combating mcr-mediated colistin resistance requires global surveillance, coordination across sectors, and continued research to stop its spread and impact.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Colistin/pharmacology
*Anti-Bacterial Agents/pharmacology
Humans
*Drug Resistance, Bacterial/genetics
Evolution, Molecular
Animals
*Gram-Negative Bacteria/genetics/drug effects
*Bacterial Proteins/genetics/metabolism
Gene Transfer, Horizontal
Plasmids/genetics
Multigene Family
Escherichia coli Proteins
RevDate: 2026-02-12
Parasitic Plant-Host Interactions: Molecular Mechanisms and Agricultural Resistance Strategies.
Advanced science (Weinheim, Baden-Wurttemberg, Germany) [Epub ahead of print].
Obligate parasitic plants, particularly members of the Orobanchaceae family, including Striga and Orobanche, greatly devastate crop production. Here, we synthesize recent advances in understanding the molecular and ecological dynamics underlying parasitic plant-host interactions, focusing on critical stages of parasitism: germination, host detection, haustorium formation, and resource extraction. Orobanchaceous parasites exploit host-derived strigolactones (SLs) to break seed dormancy, whereas Cuscuta species do not rely on SLs for germination. Instead, chemotropic responses to host-exuded compounds and light signals guide the directional growth of their seedlings. Haustorium morphogenesis, initiated through host lignin-derived quinones and redox-sensitive compounds, establishes vascular connectivity enabling nutrient diversion. Meanwhile, host organisms employ sophisticated multi-tier defense strategies encompassing SL biosynthesis, lignin deposition enhancement, hypersensitive cellular responses, and hormone-coordinated immunity. Key discoveries, such as receptor kinases and horizontal gene transfer events, highlight evolutionary arms races between parasites and hosts. Emerging technologies like CRISPR offer promising avenues for engineering resistant crops by disrupting parasitic signaling or enhancing host immunity. This review underscores the importance of integrating molecular insights with agricultural innovation to mitigate yield losses and addresses future challenges, including climate-driven parasite spread and the need for sustainable, genomics-driven solutions. By deciphering the silent dialogue between parasites and hosts, this work provides foundations for transformative strategies to safeguard global food security.
Additional Links: PMID-41677413
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41677413,
year = {2026},
author = {Shi, J and Xie, Q and Yu, F},
title = {Parasitic Plant-Host Interactions: Molecular Mechanisms and Agricultural Resistance Strategies.},
journal = {Advanced science (Weinheim, Baden-Wurttemberg, Germany)},
volume = {},
number = {},
pages = {e19030},
doi = {10.1002/advs.202519030},
pmid = {41677413},
issn = {2198-3844},
support = {2023YFF1001400//National Key Research and Development Program of China/ ; 32525045//National Natural Science Foundation of China/ ; 32222010//National Natural Science Foundation of China/ ; 32401872//National Natural Science Foundation of China/ ; 20240484588//Beijing Nova Program/ ; 2024BBF02001//Ningxia Hui Autonomous Region Key R&D Program/ ; PC2024B01004//Pinduoduo-China Agricultural University Research Fund/ ; 1201-15055009//Chinese Universities Scientific Fund/ ; },
abstract = {Obligate parasitic plants, particularly members of the Orobanchaceae family, including Striga and Orobanche, greatly devastate crop production. Here, we synthesize recent advances in understanding the molecular and ecological dynamics underlying parasitic plant-host interactions, focusing on critical stages of parasitism: germination, host detection, haustorium formation, and resource extraction. Orobanchaceous parasites exploit host-derived strigolactones (SLs) to break seed dormancy, whereas Cuscuta species do not rely on SLs for germination. Instead, chemotropic responses to host-exuded compounds and light signals guide the directional growth of their seedlings. Haustorium morphogenesis, initiated through host lignin-derived quinones and redox-sensitive compounds, establishes vascular connectivity enabling nutrient diversion. Meanwhile, host organisms employ sophisticated multi-tier defense strategies encompassing SL biosynthesis, lignin deposition enhancement, hypersensitive cellular responses, and hormone-coordinated immunity. Key discoveries, such as receptor kinases and horizontal gene transfer events, highlight evolutionary arms races between parasites and hosts. Emerging technologies like CRISPR offer promising avenues for engineering resistant crops by disrupting parasitic signaling or enhancing host immunity. This review underscores the importance of integrating molecular insights with agricultural innovation to mitigate yield losses and addresses future challenges, including climate-driven parasite spread and the need for sustainable, genomics-driven solutions. By deciphering the silent dialogue between parasites and hosts, this work provides foundations for transformative strategies to safeguard global food security.},
}
RevDate: 2026-02-12
CmpDate: 2026-02-12
Ecological context structures duplication and mobilization of antibiotic and metal resistance genes in bacteria.
bioRxiv : the preprint server for biology pii:2026.02.03.703612.
Antibiotic resistance is a global challenge driven by the persistence and spread of resistance genes across ecological contexts. While mobile genetic elements (MGEs) facilitate horizontal gene transfer, gene duplication represents an additional mechanism through which resistance genes can be amplified, diversified, and maintained under selection. How these processes interact across environments remains poorly understood. Here, we examined genome-level patterns of resistance gene abundance, duplication, and mobilization across clinical, agricultural, and wastewater settings, focusing on both antibiotic resistance genes (ARGs) and metal resistance genes (MRGs). Resistance gene profiles were strongly structured by environment, with distinct duplication patterns emerging across sources. Duplicate genes were frequently associated with MGEs, although the strength of this relationship varied by resistance type and ecological context. Despite frequent co-occurrence of ARGs and MRGs, their duplication and mobilization dynamics were not uniformly coupled at the genome level. Together, these findings highlight gene duplication as a context-dependent contributor to resistance evolution and underscore the importance of ecological setting in shaping how resistance genes persist and spread across microbial communities.
Additional Links: PMID-41676731
Full Text:
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41676731,
year = {2026},
author = {Tran, E and Xu, PN and Assis, R},
title = {Ecological context structures duplication and mobilization of antibiotic and metal resistance genes in bacteria.},
journal = {bioRxiv : the preprint server for biology},
volume = {},
number = {},
pages = {},
doi = {10.64898/2026.02.03.703612},
pmid = {41676731},
issn = {2692-8205},
abstract = {Antibiotic resistance is a global challenge driven by the persistence and spread of resistance genes across ecological contexts. While mobile genetic elements (MGEs) facilitate horizontal gene transfer, gene duplication represents an additional mechanism through which resistance genes can be amplified, diversified, and maintained under selection. How these processes interact across environments remains poorly understood. Here, we examined genome-level patterns of resistance gene abundance, duplication, and mobilization across clinical, agricultural, and wastewater settings, focusing on both antibiotic resistance genes (ARGs) and metal resistance genes (MRGs). Resistance gene profiles were strongly structured by environment, with distinct duplication patterns emerging across sources. Duplicate genes were frequently associated with MGEs, although the strength of this relationship varied by resistance type and ecological context. Despite frequent co-occurrence of ARGs and MRGs, their duplication and mobilization dynamics were not uniformly coupled at the genome level. Together, these findings highlight gene duplication as a context-dependent contributor to resistance evolution and underscore the importance of ecological setting in shaping how resistance genes persist and spread across microbial communities.},
}
RevDate: 2026-02-12
CmpDate: 2026-02-12
Systematic characterization of horizontally transferred biosynthetic gene clusters in the human gut microbiota using HTBGC-Finder.
iMetaOmics, 2(1):e62.
The human gut microbiota contains biosynthetic gene clusters (BGCs) that encode bioactive secondary metabolites, which play pivotal roles in microbe-microbe and host-microbe interactions and serve as a rich source of pharmaceutical lead compounds. Understanding the horizontal transfer of BGCs can reveal insights into microbial adaptation, resource utilization, and evolutionary mechanisms, thereby advancing biotechnological applications. Despite its importance, horizontal transfer of BGCs within the gut microbiota remains poorly understood. In this study, we introduce a novel tool, the Horizontally Transferred Biosynthetic Gene Clusters Finder (HTBGC-Finder), designed to systematically identify potential horizontally transferred BGCs (HTBGCs) within the extensive human gut microbiota. Using HTBGC-Finder, we identified 81 potential HTBGCs, underscoring the prevalence and significance of horizontal gene transfer in shaping the genetic landscape of the gut microbiome. Remarkably, ribosomally synthesized and post-translationally modified peptides (RiPPs) constituted the majority of these HTBGCs (76 out of 81, 93.83%), exhibiting a significantly higher transfer rate compared to non-RiPPs (Chi-squared test, p < 0.001). Upon detailed examination of BGCs, cyclic-lactone-autoinducer (CLA) and RiPP recognition element (RRE)-containing BGCs were predominant, representing nearly three-quarters of the total (45, or 55.56%, and 14, or 17.28%, respectively). Notably, CLA BGCs also demonstrated a higher transfer rate than non-CLA BGCs (Chi-squared test, p < 0.001). Taxonomy profiling revealed that horizontal BGC transfer occurred exclusively in the phyla Bacteroidota (synonym Bacteroidetes) and Bacillota (synonym Firmicutes), with 50 and 31 instances, respectively. Furthermore, cross-phylum transfer events were observed, highlighting the complex interactions between the gut microbiota and host health. These findings offer valuable insights into the horizontal transfer dynamics of BGCs within the gut microbiome and their potential implications for host-microbiota interactions.
Additional Links: PMID-41675707
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41675707,
year = {2025},
author = {Wu, J and Yang, X and Zhao, L and Li, Z and Zhao, G and Zhang, L},
title = {Systematic characterization of horizontally transferred biosynthetic gene clusters in the human gut microbiota using HTBGC-Finder.},
journal = {iMetaOmics},
volume = {2},
number = {1},
pages = {e62},
pmid = {41675707},
issn = {2996-9514},
abstract = {The human gut microbiota contains biosynthetic gene clusters (BGCs) that encode bioactive secondary metabolites, which play pivotal roles in microbe-microbe and host-microbe interactions and serve as a rich source of pharmaceutical lead compounds. Understanding the horizontal transfer of BGCs can reveal insights into microbial adaptation, resource utilization, and evolutionary mechanisms, thereby advancing biotechnological applications. Despite its importance, horizontal transfer of BGCs within the gut microbiota remains poorly understood. In this study, we introduce a novel tool, the Horizontally Transferred Biosynthetic Gene Clusters Finder (HTBGC-Finder), designed to systematically identify potential horizontally transferred BGCs (HTBGCs) within the extensive human gut microbiota. Using HTBGC-Finder, we identified 81 potential HTBGCs, underscoring the prevalence and significance of horizontal gene transfer in shaping the genetic landscape of the gut microbiome. Remarkably, ribosomally synthesized and post-translationally modified peptides (RiPPs) constituted the majority of these HTBGCs (76 out of 81, 93.83%), exhibiting a significantly higher transfer rate compared to non-RiPPs (Chi-squared test, p < 0.001). Upon detailed examination of BGCs, cyclic-lactone-autoinducer (CLA) and RiPP recognition element (RRE)-containing BGCs were predominant, representing nearly three-quarters of the total (45, or 55.56%, and 14, or 17.28%, respectively). Notably, CLA BGCs also demonstrated a higher transfer rate than non-CLA BGCs (Chi-squared test, p < 0.001). Taxonomy profiling revealed that horizontal BGC transfer occurred exclusively in the phyla Bacteroidota (synonym Bacteroidetes) and Bacillota (synonym Firmicutes), with 50 and 31 instances, respectively. Furthermore, cross-phylum transfer events were observed, highlighting the complex interactions between the gut microbiota and host health. These findings offer valuable insights into the horizontal transfer dynamics of BGCs within the gut microbiome and their potential implications for host-microbiota interactions.},
}
RevDate: 2026-02-11
Conjugation frequency of ESBL- and pAmpC- E. coli in broiler chickens in vivo and in vitro.
BMC microbiology pii:10.1186/s12866-026-04822-1 [Epub ahead of print].
BACKGROUND: Plasmid-mediated conjugation is a major form of horizontal gene transfer (HGT), facilitating dissemination of antimicrobial resistance (AMR) and the emergence of multi-drug-resistant (MDR) strains. In poultry, Escherichia coli producing extended-spectrum β-lactamases (ESBL) and plasmid-mediated AmpC β-lactamase (pAmpC) enzymes are common and contribute to antibiotic resistance. Additionally, plasmid-mediated colistin resistance gene mcr-1 in poultry requires attention, as it is a last-resort antibiotic in human medicine. Although plasmid-mediated conjugation is known to play a role in spreading antimicrobial resistance, its specific impact on resistance transmission within the broiler microbiota is still not well understood. We assessed conjugation dynamics of the mcr-1 gene from a pAmpC- E. coli to an ESBL- E. coli observed in an in vivo broiler chicken trial and compared them to conjugation frequencies under different in vitro conditions (LB broth, intestinal chicken cells in DMEM/F-12 medium, and DMEM/F-12 medium alone), with two initial bacterial loads.
RESULTS: From in vivo trial, among 138 broiler chickens sampled after a 49-day fattening period, transconjugants were detected in the cecal content of 35 broilers. The median conjugation frequency observed was of -5.02 log10 transconjugants/donor. Median conjugation frequencies across all in vitro conditions varied by less than one log unit (between - 6.8 and - 6.0 log10 transconjugants/donor), and no significant differences in conjugation efficiency were observed between initial bacterial concentrations.
CONCLUSIONS: We confirmed bacterial conjugation between pAmpC-producing E. coli carrying the mcr-1 gene and ESBL-producing E. coli both in vitro and in vivo. The similar conjugation efficiencies observed across different in vitro methods suggest that experimental conditions have minimal influence under controlled settings. In contrast, the in vivo results underscore the significance of the host's physiological environment in HGT. The presence of transconjugants after a 49-day fattening period indicates that intestinal bacteria function as reservoirs for resistance plasmids and could facilitate their spread throughout the broiler production chain. However, limitations like the possibility of plasmid transfer to other bacteria, unknown persistence of the plasmid in the gut, and potential modulations of transfer efficiency under antibiotic selection must be considered when interpreting the results.
Additional Links: PMID-41673559
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41673559,
year = {2026},
author = {Vargas, D and Merle, R and Friese, A and Roesler, U and Robé, C},
title = {Conjugation frequency of ESBL- and pAmpC- E. coli in broiler chickens in vivo and in vitro.},
journal = {BMC microbiology},
volume = {},
number = {},
pages = {},
doi = {10.1186/s12866-026-04822-1},
pmid = {41673559},
issn = {1471-2180},
abstract = {BACKGROUND: Plasmid-mediated conjugation is a major form of horizontal gene transfer (HGT), facilitating dissemination of antimicrobial resistance (AMR) and the emergence of multi-drug-resistant (MDR) strains. In poultry, Escherichia coli producing extended-spectrum β-lactamases (ESBL) and plasmid-mediated AmpC β-lactamase (pAmpC) enzymes are common and contribute to antibiotic resistance. Additionally, plasmid-mediated colistin resistance gene mcr-1 in poultry requires attention, as it is a last-resort antibiotic in human medicine. Although plasmid-mediated conjugation is known to play a role in spreading antimicrobial resistance, its specific impact on resistance transmission within the broiler microbiota is still not well understood. We assessed conjugation dynamics of the mcr-1 gene from a pAmpC- E. coli to an ESBL- E. coli observed in an in vivo broiler chicken trial and compared them to conjugation frequencies under different in vitro conditions (LB broth, intestinal chicken cells in DMEM/F-12 medium, and DMEM/F-12 medium alone), with two initial bacterial loads.
RESULTS: From in vivo trial, among 138 broiler chickens sampled after a 49-day fattening period, transconjugants were detected in the cecal content of 35 broilers. The median conjugation frequency observed was of -5.02 log10 transconjugants/donor. Median conjugation frequencies across all in vitro conditions varied by less than one log unit (between - 6.8 and - 6.0 log10 transconjugants/donor), and no significant differences in conjugation efficiency were observed between initial bacterial concentrations.
CONCLUSIONS: We confirmed bacterial conjugation between pAmpC-producing E. coli carrying the mcr-1 gene and ESBL-producing E. coli both in vitro and in vivo. The similar conjugation efficiencies observed across different in vitro methods suggest that experimental conditions have minimal influence under controlled settings. In contrast, the in vivo results underscore the significance of the host's physiological environment in HGT. The presence of transconjugants after a 49-day fattening period indicates that intestinal bacteria function as reservoirs for resistance plasmids and could facilitate their spread throughout the broiler production chain. However, limitations like the possibility of plasmid transfer to other bacteria, unknown persistence of the plasmid in the gut, and potential modulations of transfer efficiency under antibiotic selection must be considered when interpreting the results.},
}
RevDate: 2026-02-11
The Gut Microbiome as a Modulator of Antibiotic Resistance: Mechanisms, Dynamics, and Therapeutic Interventions.
Microbial pathogenesis pii:S0882-4010(26)00083-5 [Epub ahead of print].
The gut microbiome is increasingly recognized as a critical factor in the dynamics of antibiotic resistance, influencing the acquisition, persistence, and dissemination of antibiotic resistance genes (ARGs) among both commensal and pathogenic bacteria. This research focuses on elucidating the mechanisms by which the gut microbiome modulates the horizontal gene transfer (HGT) of ARGs, a key driver of the global antibiotic resistance crisis. By employing advanced metagenomic sequencing and functional assays, this study aims to identify specific microbial species, genetic elements, and metabolic pathways that either facilitate or inhibit the transfer of ARGs within the gut environment. Particular attention is given to the role of microbial metabolites, interspecies interactions, and environmental factors that shape the resistome the collection of all resistance genes within the microbiome. Additionally, this research explores innovative microbiome-based interventions, such as the use of probiotics, prebiotics, and bacteriophage therapy, to disrupt the transmission of ARGs and restore microbial balance. These interventions are designed to target the gut microbiome as a reservoir of resistance genes, offering a novel approach to curbing the spread of antibiotic resistance. The significance of this work lies in its potential to provide actionable insights into microbiome-mediated resistance mechanisms and to develop targeted strategies that complement traditional antibiotic therapies. By addressing the gut microbiome as a modifiable factor in the resistance landscape, this research could contribute to mitigating the global burden of antibiotic resistance, preserving the efficacy of existing treatments, and improving public health outcomes in the face of this pressing challenge.
Additional Links: PMID-41672331
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41672331,
year = {2026},
author = {Yu, J and Allela, OQB and Alkhazali, WH and Bishoyi, AK and Oweis, R and Varma, P and Kashyap, A and Panigrahi, R and Chauhan, AS and Sameer, HN and Yaseen, A and Athab, ZH and Adil, M},
title = {The Gut Microbiome as a Modulator of Antibiotic Resistance: Mechanisms, Dynamics, and Therapeutic Interventions.},
journal = {Microbial pathogenesis},
volume = {},
number = {},
pages = {108357},
doi = {10.1016/j.micpath.2026.108357},
pmid = {41672331},
issn = {1096-1208},
abstract = {The gut microbiome is increasingly recognized as a critical factor in the dynamics of antibiotic resistance, influencing the acquisition, persistence, and dissemination of antibiotic resistance genes (ARGs) among both commensal and pathogenic bacteria. This research focuses on elucidating the mechanisms by which the gut microbiome modulates the horizontal gene transfer (HGT) of ARGs, a key driver of the global antibiotic resistance crisis. By employing advanced metagenomic sequencing and functional assays, this study aims to identify specific microbial species, genetic elements, and metabolic pathways that either facilitate or inhibit the transfer of ARGs within the gut environment. Particular attention is given to the role of microbial metabolites, interspecies interactions, and environmental factors that shape the resistome the collection of all resistance genes within the microbiome. Additionally, this research explores innovative microbiome-based interventions, such as the use of probiotics, prebiotics, and bacteriophage therapy, to disrupt the transmission of ARGs and restore microbial balance. These interventions are designed to target the gut microbiome as a reservoir of resistance genes, offering a novel approach to curbing the spread of antibiotic resistance. The significance of this work lies in its potential to provide actionable insights into microbiome-mediated resistance mechanisms and to develop targeted strategies that complement traditional antibiotic therapies. By addressing the gut microbiome as a modifiable factor in the resistance landscape, this research could contribute to mitigating the global burden of antibiotic resistance, preserving the efficacy of existing treatments, and improving public health outcomes in the face of this pressing challenge.},
}
RevDate: 2026-02-11
Persulfates radical-driven advanced oxidation: promising approach to regulate antibiotic resistance genes in composting systems.
Bioresource technology pii:S0960-8524(26)00271-3 [Epub ahead of print].
Composting serves as a pivotal technology for recycling livestock manure and reducing antibiotic resistance genes (ARGs). However, optimizing only its physicochemical properties or microbial community yields limited success in ARG removal. In contrast, persulfate radical-driven advanced oxidation processes (AOPs) have proven highly effective in eliminating ARGs. This study demonstrates that the biological heat generated during composting can activates persulfate, not only boosting the ARGs removal rate to 96% but also effectively suppressing the rebound and re-enrichment of ARGs during the compost maturation stage, maintaining a removal rate of 55%. Specifically, this approach reduces the abundances of mobile genetic elements (MGEs, e.g., intI2, IncQ-oriV) and target ARGs (tetA, tetQ, strA, sul3). The mechanisms underlying ARG removal involve two key aspects: First, strong oxidative radicals produced by persulfate activation directly oxidize and damage resistant bacteria, thereby decreasing the abundances of ARGs and MGEs. Second, persulfate primarily inhibits ARGs transmission by reshaping the bacterial community structure. In traditional composting, non-host core bacteria act as "bridges" connecting distinct microbial modules, directly facilitating inter-modular ARGs transmission. Dominant genera such as Bacillus, norank_f__Limnochordaceae, Marinimicrobium, and Tepidimicrobium mainly carry key MGEs (intI2, Tn916/1545, tnpA, IS613), which further amplify the risk of ARGs dissemination. In contrast, following persulfate addition, only Truepera is detected as a non-host core bacterium, significantly reducing cross-module ARGs transmission pathways. This study offers a promising regulation strategy for mitigating ARG-related risks during composting.
Additional Links: PMID-41672316
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41672316,
year = {2026},
author = {Wang, J and Gao, X and Wei, N and Ma, R and Yang, Y and Li, G and Tian, Y and Yuan, J},
title = {Persulfates radical-driven advanced oxidation: promising approach to regulate antibiotic resistance genes in composting systems.},
journal = {Bioresource technology},
volume = {},
number = {},
pages = {134190},
doi = {10.1016/j.biortech.2026.134190},
pmid = {41672316},
issn = {1873-2976},
abstract = {Composting serves as a pivotal technology for recycling livestock manure and reducing antibiotic resistance genes (ARGs). However, optimizing only its physicochemical properties or microbial community yields limited success in ARG removal. In contrast, persulfate radical-driven advanced oxidation processes (AOPs) have proven highly effective in eliminating ARGs. This study demonstrates that the biological heat generated during composting can activates persulfate, not only boosting the ARGs removal rate to 96% but also effectively suppressing the rebound and re-enrichment of ARGs during the compost maturation stage, maintaining a removal rate of 55%. Specifically, this approach reduces the abundances of mobile genetic elements (MGEs, e.g., intI2, IncQ-oriV) and target ARGs (tetA, tetQ, strA, sul3). The mechanisms underlying ARG removal involve two key aspects: First, strong oxidative radicals produced by persulfate activation directly oxidize and damage resistant bacteria, thereby decreasing the abundances of ARGs and MGEs. Second, persulfate primarily inhibits ARGs transmission by reshaping the bacterial community structure. In traditional composting, non-host core bacteria act as "bridges" connecting distinct microbial modules, directly facilitating inter-modular ARGs transmission. Dominant genera such as Bacillus, norank_f__Limnochordaceae, Marinimicrobium, and Tepidimicrobium mainly carry key MGEs (intI2, Tn916/1545, tnpA, IS613), which further amplify the risk of ARGs dissemination. In contrast, following persulfate addition, only Truepera is detected as a non-host core bacterium, significantly reducing cross-module ARGs transmission pathways. This study offers a promising regulation strategy for mitigating ARG-related risks during composting.},
}
RevDate: 2026-02-11
Evolutionary mechanisms underlying bacterial adaptation to the plant environment.
FEMS microbiology reviews pii:8472863 [Epub ahead of print].
Plants and bacteria have coevolved over hundreds of millions of years, forming complex associations ranging from mutualism to pathogenicity that are essential for plant survival and ecosystem function. Bacterial adaptation to plant environments involves dynamic evolutionary mechanisms including horizontal gene transfer, gene regulation, and metabolic specialization, enabling bacteria to persist and specialize within diverse plant-associated niches. Here we review how evolutionary forces such as selection, drift, and gene flow shape bacterial genomes, regulatory networks, and ecological strategies in response to plant-imposed pressures, underpinning both beneficial and pathogenic lifestyles. Understanding these processes provides a unified evolutionary framework for bacterial adaptation to plants and highlights their implications for sustainable agriculture and microbiome-based innovations.
Additional Links: PMID-41671169
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41671169,
year = {2026},
author = {Saati-Santamaría, Z and Pérez-Mendoza, D and Khashi U Rahman, M and de Sousa, BFS and Montero-Calasanz, MDC and Rey, L and Roy, S and Sanjuán, J and García-Fraile, P},
title = {Evolutionary mechanisms underlying bacterial adaptation to the plant environment.},
journal = {FEMS microbiology reviews},
volume = {},
number = {},
pages = {},
doi = {10.1093/femsre/fuag005},
pmid = {41671169},
issn = {1574-6976},
abstract = {Plants and bacteria have coevolved over hundreds of millions of years, forming complex associations ranging from mutualism to pathogenicity that are essential for plant survival and ecosystem function. Bacterial adaptation to plant environments involves dynamic evolutionary mechanisms including horizontal gene transfer, gene regulation, and metabolic specialization, enabling bacteria to persist and specialize within diverse plant-associated niches. Here we review how evolutionary forces such as selection, drift, and gene flow shape bacterial genomes, regulatory networks, and ecological strategies in response to plant-imposed pressures, underpinning both beneficial and pathogenic lifestyles. Understanding these processes provides a unified evolutionary framework for bacterial adaptation to plants and highlights their implications for sustainable agriculture and microbiome-based innovations.},
}
RevDate: 2026-02-11
The Influence of National Antibiotic Consumption on Neisseria gonorrhoeae Antibiotic Resistance in Norway, 2003-2024.
The Journal of infectious diseases pii:8469279 [Epub ahead of print].
OBJECTIVES: To investigate whether national population-level antibiotic consumption influences antimicrobial resistance (AMR) in Norwegian Neisseria gonorrhoeae isolates. To explore metrics suitable for ecological AMR studies.
METHODS: Longitudinal Norwegian gonococcal susceptibility data (2003-2024) were analysed alongside national antibiotic consumption. Temporal trends were examined graphically and associations assessed using one-tailed Spearman rank correlations. Novel metrics - The 'Susceptible Isolate Pressure Indicator' (SIPI) and 'Wild-Type Isolate Pressure Indicator' (WIPI) ratios were introduced to characterise shifts in minimum inhibitory concentration (MIC) distributions within susceptible or wild-type ranges.
RESULTS: Strong positive, significant correlations were observed between consumption of the most widely used antibiotic classes in Norway - betalactamase-sensitive penicillins and tetracyclines - and gonococcal geometric mean MIC for benzylpenicillin (ρ=0.776, p<0.001) and tetracycline (ρ = 0.841, p<0.001). Penicillin-class consumption was also significantly associated with betalactamase plasmid carriage (ρ = 0.637, p = 0.013), consistent with horizontal gene transfer from commensal flora.
CONCLUSIONS: Even in a low-consumption European context, Norwegian antibiotic use appears to shape gonococcal resistance, possibly partly via gene uptake from commensal Neisseria. The SIPI and WIPI ratios describe susceptible-range MIC histogram shapes, and offer utility for AMR surveillance by capturing isolate flux.
Additional Links: PMID-41670362
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41670362,
year = {2026},
author = {Campbell, P},
title = {The Influence of National Antibiotic Consumption on Neisseria gonorrhoeae Antibiotic Resistance in Norway, 2003-2024.},
journal = {The Journal of infectious diseases},
volume = {},
number = {},
pages = {},
doi = {10.1093/infdis/jiag076},
pmid = {41670362},
issn = {1537-6613},
abstract = {OBJECTIVES: To investigate whether national population-level antibiotic consumption influences antimicrobial resistance (AMR) in Norwegian Neisseria gonorrhoeae isolates. To explore metrics suitable for ecological AMR studies.
METHODS: Longitudinal Norwegian gonococcal susceptibility data (2003-2024) were analysed alongside national antibiotic consumption. Temporal trends were examined graphically and associations assessed using one-tailed Spearman rank correlations. Novel metrics - The 'Susceptible Isolate Pressure Indicator' (SIPI) and 'Wild-Type Isolate Pressure Indicator' (WIPI) ratios were introduced to characterise shifts in minimum inhibitory concentration (MIC) distributions within susceptible or wild-type ranges.
RESULTS: Strong positive, significant correlations were observed between consumption of the most widely used antibiotic classes in Norway - betalactamase-sensitive penicillins and tetracyclines - and gonococcal geometric mean MIC for benzylpenicillin (ρ=0.776, p<0.001) and tetracycline (ρ = 0.841, p<0.001). Penicillin-class consumption was also significantly associated with betalactamase plasmid carriage (ρ = 0.637, p = 0.013), consistent with horizontal gene transfer from commensal flora.
CONCLUSIONS: Even in a low-consumption European context, Norwegian antibiotic use appears to shape gonococcal resistance, possibly partly via gene uptake from commensal Neisseria. The SIPI and WIPI ratios describe susceptible-range MIC histogram shapes, and offer utility for AMR surveillance by capturing isolate flux.},
}
RevDate: 2026-02-11
CmpDate: 2026-02-11
The role of uropathogenic Escherichia coli biofilms in antibiotic-resistant urinary tract infections: Nanoparticle-based, phage therapy, and quorum-sensing inhibitor approaches.
Current urology, 20(2):82-88.
BACKGROUND: Urinary tract infections (UTIs) caused by uropathogenic E. coli (UPEC) pose a global health challenge, largely due to UPEC biofilms that drive persistent infections and antibiotic resistance.
MATERIALS AND METHODS: To explore the role of UPEC biofilms in antibiotic-resistant UTIs and summarize emerging therapeutic strategies, this study conducted a systematic review adhering to PRISMA guidelines and registered in PROSPERO (CRD420251040212). A structured search of PubMed, Google Scholar, Scopus, and Web of Science identified English-language studies published up to 2024, with 57 eligible studies selected after three-stage screening and analyzed via thematic synthesis.
RESULTS: This study explored UPEC biofilms enhance resistance through extracellular matrix barriers, persister cell formation, efflux pump upregulation, and horizontal gene transfer; emerging therapies including bacteriophage therapy, quorum-sensing inhibitors, and nanoparticle-based drug delivery effectively target biofilms by penetration, signaling disruption, and improved drug efficacy. Additional approaches such as antibiofilm peptides, probiotics, and immunotherapy also demonstrate potential.
CONCLUSIONS: The UPEC biofilms are key to chronic UTIs, and novel targeted therapies offer promising solutions, but clinical validation, regulatory hurdles, and combination therapy optimization are critical for translation to clinical practice.
Additional Links: PMID-41668896
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41668896,
year = {2026},
author = {Eskandar, K},
title = {The role of uropathogenic Escherichia coli biofilms in antibiotic-resistant urinary tract infections: Nanoparticle-based, phage therapy, and quorum-sensing inhibitor approaches.},
journal = {Current urology},
volume = {20},
number = {2},
pages = {82-88},
pmid = {41668896},
issn = {1661-7649},
abstract = {BACKGROUND: Urinary tract infections (UTIs) caused by uropathogenic E. coli (UPEC) pose a global health challenge, largely due to UPEC biofilms that drive persistent infections and antibiotic resistance.
MATERIALS AND METHODS: To explore the role of UPEC biofilms in antibiotic-resistant UTIs and summarize emerging therapeutic strategies, this study conducted a systematic review adhering to PRISMA guidelines and registered in PROSPERO (CRD420251040212). A structured search of PubMed, Google Scholar, Scopus, and Web of Science identified English-language studies published up to 2024, with 57 eligible studies selected after three-stage screening and analyzed via thematic synthesis.
RESULTS: This study explored UPEC biofilms enhance resistance through extracellular matrix barriers, persister cell formation, efflux pump upregulation, and horizontal gene transfer; emerging therapies including bacteriophage therapy, quorum-sensing inhibitors, and nanoparticle-based drug delivery effectively target biofilms by penetration, signaling disruption, and improved drug efficacy. Additional approaches such as antibiofilm peptides, probiotics, and immunotherapy also demonstrate potential.
CONCLUSIONS: The UPEC biofilms are key to chronic UTIs, and novel targeted therapies offer promising solutions, but clinical validation, regulatory hurdles, and combination therapy optimization are critical for translation to clinical practice.},
}
RevDate: 2026-02-11
CmpDate: 2026-02-11
Genome-based characterization of AHPND and non-AHPND Vibrio campbellii isolates from Republic of Korea.
Frontiers in microbiology, 17:1724818.
With mounting evidence that Vibrio campbellii can act as a causative agent, acute hepatopancreatic necrosis disease (AHPND) represents a serious threat to global shrimp aquaculture. In this study, we present a comparative genomic analysis of 101 V. campbellii strains, including the recently isolated pathogenic and non-pathogenic strains, V. campbellii HJ-2023 and V. campbellii HJ-2023n, from the Republic of Korea. Whole-genome sequencing revealed that the pathogenic strain harbors three plasmids and carries the canonical AHPND toxin genes pirA and pirB, along with an expanded repertoire of virulence and secretion system genes. Pan-genome and insertion sequence analyses showed that pathogenic strains tend to cluster based on shared mobile genetic elements, particularly transposases located near toxin genes, underscoring the role of horizontal gene transfer in virulence acquisition. Although all strains displayed a broad distribution of antibiotic-resistance genes, pathogenicity did not consistently correlate with their presence. Similarly, carbohydrate-active enzyme (CAZyme) profiles were largely conserved, although certain enzymes, such as chitinases, may contribute accessory functions in host invasion. Notably, the AHPND-associated V. campbellii HJ-2023 strain contained multiple copies of key T6SS and T1SS genes, suggesting an increased potential for toxin delivery. These findings suggest that pathogenic potential in V. campbellii likely arises not only from the presence of toxins but also from the complex interplay of mobile elements, secretion systems, and genomic architecture. This study provides an essential genomic framework for understanding the emergence of AHPND in V. campbellii and offers insights to enhance molecular diagnostics, strengthen biosecurity, and improve disease control strategies in shrimp aquaculture.
Additional Links: PMID-41668884
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41668884,
year = {2026},
author = {Lee, SY and Choi, HJ and Lee, S and Choi, J and Kwak, JS and Kang, YJ and Hong, SC},
title = {Genome-based characterization of AHPND and non-AHPND Vibrio campbellii isolates from Republic of Korea.},
journal = {Frontiers in microbiology},
volume = {17},
number = {},
pages = {1724818},
pmid = {41668884},
issn = {1664-302X},
abstract = {With mounting evidence that Vibrio campbellii can act as a causative agent, acute hepatopancreatic necrosis disease (AHPND) represents a serious threat to global shrimp aquaculture. In this study, we present a comparative genomic analysis of 101 V. campbellii strains, including the recently isolated pathogenic and non-pathogenic strains, V. campbellii HJ-2023 and V. campbellii HJ-2023n, from the Republic of Korea. Whole-genome sequencing revealed that the pathogenic strain harbors three plasmids and carries the canonical AHPND toxin genes pirA and pirB, along with an expanded repertoire of virulence and secretion system genes. Pan-genome and insertion sequence analyses showed that pathogenic strains tend to cluster based on shared mobile genetic elements, particularly transposases located near toxin genes, underscoring the role of horizontal gene transfer in virulence acquisition. Although all strains displayed a broad distribution of antibiotic-resistance genes, pathogenicity did not consistently correlate with their presence. Similarly, carbohydrate-active enzyme (CAZyme) profiles were largely conserved, although certain enzymes, such as chitinases, may contribute accessory functions in host invasion. Notably, the AHPND-associated V. campbellii HJ-2023 strain contained multiple copies of key T6SS and T1SS genes, suggesting an increased potential for toxin delivery. These findings suggest that pathogenic potential in V. campbellii likely arises not only from the presence of toxins but also from the complex interplay of mobile elements, secretion systems, and genomic architecture. This study provides an essential genomic framework for understanding the emergence of AHPND in V. campbellii and offers insights to enhance molecular diagnostics, strengthen biosecurity, and improve disease control strategies in shrimp aquaculture.},
}
RevDate: 2026-02-10
Planetary microbiome structure and generalist-driven gene flow across disparate habitats.
Cell pii:S0092-8674(25)01500-4 [Epub ahead of print].
Microbes are ubiquitous on Earth, forming microbiomes that sustain macroscopic life and biogeochemical cycles. Microbial dispersal, driven by natural processes and human activities, interconnects microbiomes across habitats, yet most comparative studies focus on specific ecosystems. To study planetary microbiome structure, function, and inter-habitat interactions, we systematically integrated 85,604 public metagenomes spanning diverse habitats worldwide. Using species-based unsupervised clustering and parameter modeling, we delineated 40 habitat clusters and quantified their ecological similarity. Our framework identified key drivers shaping microbiome structure, such as ocean temperature and host lifestyle. Regardless of biogeography, microbiomes were structured primarily by host-associated or environmental conditions, also reflected in genomic and functional traits inferred from 2,065,975 genomes. Generalists emerged as vehicles thriving and facilitating gene flow across ecologically disparate habitat types, illustrated by generalist-mediated horizontal transfer of an antibiotic resistance island across human gut and wastewater, further dispersing to environmental habitats, exemplifying human impact on the planetary microbiome.
Additional Links: PMID-41666926
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41666926,
year = {2026},
author = {Kim, CY and Podlesny, D and Schiller, J and Khedkar, S and Fullam, A and Orakov, A and Schudoma, C and Robbani, SM and Grekova, A and Kuhn, M and Bork, P},
title = {Planetary microbiome structure and generalist-driven gene flow across disparate habitats.},
journal = {Cell},
volume = {},
number = {},
pages = {},
doi = {10.1016/j.cell.2025.12.051},
pmid = {41666926},
issn = {1097-4172},
abstract = {Microbes are ubiquitous on Earth, forming microbiomes that sustain macroscopic life and biogeochemical cycles. Microbial dispersal, driven by natural processes and human activities, interconnects microbiomes across habitats, yet most comparative studies focus on specific ecosystems. To study planetary microbiome structure, function, and inter-habitat interactions, we systematically integrated 85,604 public metagenomes spanning diverse habitats worldwide. Using species-based unsupervised clustering and parameter modeling, we delineated 40 habitat clusters and quantified their ecological similarity. Our framework identified key drivers shaping microbiome structure, such as ocean temperature and host lifestyle. Regardless of biogeography, microbiomes were structured primarily by host-associated or environmental conditions, also reflected in genomic and functional traits inferred from 2,065,975 genomes. Generalists emerged as vehicles thriving and facilitating gene flow across ecologically disparate habitat types, illustrated by generalist-mediated horizontal transfer of an antibiotic resistance island across human gut and wastewater, further dispersing to environmental habitats, exemplifying human impact on the planetary microbiome.},
}
RevDate: 2026-02-10
Heating-season dynamics of the airborne microbiome, resistome and mobilome in Belgrade, Serbia.
Environment international, 208:110114 pii:S0160-4120(26)00072-3 [Epub ahead of print].
Antimicrobial resistance (AMR) and air pollution are critical global health challenges, but their interplay remains poorly understood, particularly in Europe. Serbia, characterized by extensive antibiotic use, high prevalence of multidrug-resistant isolates and severe air pollution, provides a relevant model to study airborne AMR dissemination. During the heating season, air samples were collected at eight locations in Belgrade, representing industrial, traffic loaded and background environments. Shotgun metagenomics, co-occurrence networks and NMDS ordinations were applied to investigate the relationships between atmospheric pollutants, antibiotic resistance genes (ARGs), biocide resistance genes (BRGs), metal resistance genes (MRGs) and mobile genetic elements (MGEs). Autumn microbiomes were dominated by Lactococcus spp., whereas winter lacked such dominance. ARGs associated with antibiotic inactivation accounted for > 50% in autumn and > 75% in winter, with β-lactam resistance (blaTEM) predominating in both seasons. Winter resistomes also showed more consistent patterns of BRGs and MRGs, with multibiocide/acid and multimetal resistance prevailing. Integron analysis revealed predominance of class 1 integrons (intI1) commonly associated with Escherichia coli. Plasmid-related contigs were most similar to sequences reported in Acinetobacter baumannii and E. coli, while plasmid signatures related to Lactococcus lactis were also detected in autumn. Crucially, the network analysis revealed a seasonal restructuring of the airborne resistome. Autumn networks displayed fragmented structure, showing antagonism between Lactococcus and Escherichia, whereas winter networks coalesced into a densely interconnected superhub that could facilitate horizontal gene transfer and co-selection of resistance determinants. These findings suggest that prolonged air pollution and seasonality jointly shape airborne resistomes, reinforcing the need for integrated environmental and AMR surveillance in highly polluted urban areas.
Additional Links: PMID-41666847
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41666847,
year = {2026},
author = {Matijašević, D and Kljajević, N and Malešević, M and Gardijan, L and Stanovčić, S and Jovčić, B and Novović, K},
title = {Heating-season dynamics of the airborne microbiome, resistome and mobilome in Belgrade, Serbia.},
journal = {Environment international},
volume = {208},
number = {},
pages = {110114},
doi = {10.1016/j.envint.2026.110114},
pmid = {41666847},
issn = {1873-6750},
abstract = {Antimicrobial resistance (AMR) and air pollution are critical global health challenges, but their interplay remains poorly understood, particularly in Europe. Serbia, characterized by extensive antibiotic use, high prevalence of multidrug-resistant isolates and severe air pollution, provides a relevant model to study airborne AMR dissemination. During the heating season, air samples were collected at eight locations in Belgrade, representing industrial, traffic loaded and background environments. Shotgun metagenomics, co-occurrence networks and NMDS ordinations were applied to investigate the relationships between atmospheric pollutants, antibiotic resistance genes (ARGs), biocide resistance genes (BRGs), metal resistance genes (MRGs) and mobile genetic elements (MGEs). Autumn microbiomes were dominated by Lactococcus spp., whereas winter lacked such dominance. ARGs associated with antibiotic inactivation accounted for > 50% in autumn and > 75% in winter, with β-lactam resistance (blaTEM) predominating in both seasons. Winter resistomes also showed more consistent patterns of BRGs and MRGs, with multibiocide/acid and multimetal resistance prevailing. Integron analysis revealed predominance of class 1 integrons (intI1) commonly associated with Escherichia coli. Plasmid-related contigs were most similar to sequences reported in Acinetobacter baumannii and E. coli, while plasmid signatures related to Lactococcus lactis were also detected in autumn. Crucially, the network analysis revealed a seasonal restructuring of the airborne resistome. Autumn networks displayed fragmented structure, showing antagonism between Lactococcus and Escherichia, whereas winter networks coalesced into a densely interconnected superhub that could facilitate horizontal gene transfer and co-selection of resistance determinants. These findings suggest that prolonged air pollution and seasonality jointly shape airborne resistomes, reinforcing the need for integrated environmental and AMR surveillance in highly polluted urban areas.},
}
RevDate: 2026-02-10
Host adaptation in Salmonella enterica serovar Typhimurium: population structure, pathovariants, and genomic mechanisms.
Applied and environmental microbiology [Epub ahead of print].
Salmonella enterica serovar Typhimurium is a major zoonotic pathogen of global concern to human and animal health. With its broad host range, this serovar can colonize humans as well as domesticated and wild animals. Although historically considered a model host-generalist pathogen, whole-genome sequencing (WGS) has uncovered substantial genetic diversity and the emergence of multiple host-adapted pathovariants within this serovar. In this minireview, we delineate the population structure of S. Typhimurium across diverse host species and identify the lineages/pathovariants specifically adapted to avian hosts (e.g., passerines, pigeons, ducks, geese, larids, and water birds) and those adapted to non-avian hosts (e.g., humans). We further discuss the genetic mechanisms underlying host adaptation of S. Typhimurium pathovariants, including genome degradation through point mutations and insertions/deletions, as well as the acquisition of prophages or antimicrobial resistance genes via horizontal gene transfer. The ongoing emergence of host-adapted pathovariants in zoonotic pathogens such as S. Typhimurium underscores the importance of high-resolution, WGS-based subtyping approaches for precise pathogen identification and source attribution. Moreover, elucidating the genetic mechanisms driving host adaptation of zoonotic pathogens at the strain level is essential for informing targeted strategies for surveillance, prevention, and control.
Additional Links: PMID-41665349
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41665349,
year = {2026},
author = {Gong, H and Wu, Q and Xu, M and Meng, W and Wang, Y and Ju, C and Fu, Y},
title = {Host adaptation in Salmonella enterica serovar Typhimurium: population structure, pathovariants, and genomic mechanisms.},
journal = {Applied and environmental microbiology},
volume = {},
number = {},
pages = {e0220125},
doi = {10.1128/aem.02201-25},
pmid = {41665349},
issn = {1098-5336},
abstract = {Salmonella enterica serovar Typhimurium is a major zoonotic pathogen of global concern to human and animal health. With its broad host range, this serovar can colonize humans as well as domesticated and wild animals. Although historically considered a model host-generalist pathogen, whole-genome sequencing (WGS) has uncovered substantial genetic diversity and the emergence of multiple host-adapted pathovariants within this serovar. In this minireview, we delineate the population structure of S. Typhimurium across diverse host species and identify the lineages/pathovariants specifically adapted to avian hosts (e.g., passerines, pigeons, ducks, geese, larids, and water birds) and those adapted to non-avian hosts (e.g., humans). We further discuss the genetic mechanisms underlying host adaptation of S. Typhimurium pathovariants, including genome degradation through point mutations and insertions/deletions, as well as the acquisition of prophages or antimicrobial resistance genes via horizontal gene transfer. The ongoing emergence of host-adapted pathovariants in zoonotic pathogens such as S. Typhimurium underscores the importance of high-resolution, WGS-based subtyping approaches for precise pathogen identification and source attribution. Moreover, elucidating the genetic mechanisms driving host adaptation of zoonotic pathogens at the strain level is essential for informing targeted strategies for surveillance, prevention, and control.},
}
RevDate: 2026-02-09
Efficient detection and typing of phage-plasmids.
mBio [Epub ahead of print].
UNLABELLED: Phage-plasmids (P-Ps) are temperate phages that replicate as plasmids during lysogeny. Despite their high diversity, they carry genes similar to phages and plasmids. This leads to gene exchanges and to the formation of hybrid or defective elements, which limits accurate detection of P-Ps. To address this challenge, we developed tyPPing, an easy-to-use method that efficiently detects and types P-Ps with high accuracy. It searches for distinct frequencies and sets of conserved proteins to separate P-Ps from plasmids and phages. tyPPing's strength comes from both its precise predictions and its ability to systematically type P-Ps, including the assignment of confidence levels. We tested tyPPing on several databases and a collection of incomplete (draft) genomes. While predictions rely on the quality of assemblies, we detected high-quality P-Ps and experimentally proved them to be functional. Compared to other classification methods, tyPPing is designed to detect distinct P-P types and surpasses other tools in terms of sensitivity and scalability. P-Ps are highly diverse, making the systematic identification of new types a difficult task. By combining tyPPing with other tools, however, we show a valuable foundation for addressing this challenge. How to use tyPPing and other approaches is documented in our GitHub repository: github.com/EpfeiferNutri/Phage-plasmids/.
IMPORTANCE: Mobile genetic elements, such as phages and plasmids, are diverse and drive bacterial evolution through horizontal gene transfer. Phage-plasmids, of which many carry antibiotic resistance genes or virulence factors, are both phages and plasmids and have life cycles of temperate phages and plasmids. This makes accurate classification difficult as current computational tools typically classify them as one or the other. We addressed this problem by developing tyPPing, a new and highly precise method, to systematically identify, separate, and catalog phage-plasmids. We demonstrated that tyPPing is highly accurate and broadly compatible. It provides a reliable foundation for all future studies involving phages and plasmids, ranging from agriculture environments to pathogenic strains of clinical settings.
Additional Links: PMID-41660861
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41660861,
year = {2026},
author = {Ilchenko, K and Bonnin, RA and Rocha, EPC and Pfeifer, E},
title = {Efficient detection and typing of phage-plasmids.},
journal = {mBio},
volume = {},
number = {},
pages = {e0300025},
doi = {10.1128/mbio.03000-25},
pmid = {41660861},
issn = {2150-7511},
abstract = {UNLABELLED: Phage-plasmids (P-Ps) are temperate phages that replicate as plasmids during lysogeny. Despite their high diversity, they carry genes similar to phages and plasmids. This leads to gene exchanges and to the formation of hybrid or defective elements, which limits accurate detection of P-Ps. To address this challenge, we developed tyPPing, an easy-to-use method that efficiently detects and types P-Ps with high accuracy. It searches for distinct frequencies and sets of conserved proteins to separate P-Ps from plasmids and phages. tyPPing's strength comes from both its precise predictions and its ability to systematically type P-Ps, including the assignment of confidence levels. We tested tyPPing on several databases and a collection of incomplete (draft) genomes. While predictions rely on the quality of assemblies, we detected high-quality P-Ps and experimentally proved them to be functional. Compared to other classification methods, tyPPing is designed to detect distinct P-P types and surpasses other tools in terms of sensitivity and scalability. P-Ps are highly diverse, making the systematic identification of new types a difficult task. By combining tyPPing with other tools, however, we show a valuable foundation for addressing this challenge. How to use tyPPing and other approaches is documented in our GitHub repository: github.com/EpfeiferNutri/Phage-plasmids/.
IMPORTANCE: Mobile genetic elements, such as phages and plasmids, are diverse and drive bacterial evolution through horizontal gene transfer. Phage-plasmids, of which many carry antibiotic resistance genes or virulence factors, are both phages and plasmids and have life cycles of temperate phages and plasmids. This makes accurate classification difficult as current computational tools typically classify them as one or the other. We addressed this problem by developing tyPPing, a new and highly precise method, to systematically identify, separate, and catalog phage-plasmids. We demonstrated that tyPPing is highly accurate and broadly compatible. It provides a reliable foundation for all future studies involving phages and plasmids, ranging from agriculture environments to pathogenic strains of clinical settings.},
}
RevDate: 2026-02-09
CmpDate: 2026-02-09
Shotgun metagenomics reveals the prevalence and mobility of antibiotic resistance genes in the West Bay of the human-impacted Laguna Lake.
Frontiers in microbiology, 17:1742578.
Laguna Lake, the largest freshwater lake in the Philippines, has been reported to harbor antibiotic-resistant bacteria, posing health risks to the millions who depend on it. However, limited knowledge of antibiotic resistance genes (ARGs) in the lake highlights the need for a comprehensive assessment of its resistome. In line with this, we characterized ARGs in the West Bay of Laguna Lake using shotgun metagenomic sequencing based on six metagenomes collected from three stations across two sampling months at a single depth. ARGs were quantified from short reads, and assembled contigs containing these genes-antibiotic-resistant contigs (ARCs)-were analyzed to assess mobility through associations with plasmids and mobile genetic elements (MGEs). β-lactam resistance genes (0.023-0.048 copies per cell) were the most prevalent, corroborating previous reports. Meanwhile, the detection of bacitracin (0.013-0.028 cpc) and polymyxin (0.009-0.011 cpc) resistance genes raises new concerns, as resistance to these antibiotic classes has not been previously reported in the lake. Furthermore, 44.8 and 30.4% of ARCs were associated with plasmids and MGEs, respectively. ARCs carrying genes for resistance to β-lactams, chloramphenicol, and tetracyclines were frequently identified as mobile, indicating a high potential for horizontal gene transfer and suggesting possible antibiotic contamination in the lake. Overall, this study provides the first metagenomic insight into the resistome of Laguna Lake using short-read sequencing and highlights its role as an environmental reservoir of mobile ARGs. The findings underscore the need for expanded ARG surveillance to improve antimicrobial resistance risk prediction.
Additional Links: PMID-41657901
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41657901,
year = {2026},
author = {Farinas, LMF and Dela Peña, LBRO and Rivera, WL},
title = {Shotgun metagenomics reveals the prevalence and mobility of antibiotic resistance genes in the West Bay of the human-impacted Laguna Lake.},
journal = {Frontiers in microbiology},
volume = {17},
number = {},
pages = {1742578},
pmid = {41657901},
issn = {1664-302X},
abstract = {Laguna Lake, the largest freshwater lake in the Philippines, has been reported to harbor antibiotic-resistant bacteria, posing health risks to the millions who depend on it. However, limited knowledge of antibiotic resistance genes (ARGs) in the lake highlights the need for a comprehensive assessment of its resistome. In line with this, we characterized ARGs in the West Bay of Laguna Lake using shotgun metagenomic sequencing based on six metagenomes collected from three stations across two sampling months at a single depth. ARGs were quantified from short reads, and assembled contigs containing these genes-antibiotic-resistant contigs (ARCs)-were analyzed to assess mobility through associations with plasmids and mobile genetic elements (MGEs). β-lactam resistance genes (0.023-0.048 copies per cell) were the most prevalent, corroborating previous reports. Meanwhile, the detection of bacitracin (0.013-0.028 cpc) and polymyxin (0.009-0.011 cpc) resistance genes raises new concerns, as resistance to these antibiotic classes has not been previously reported in the lake. Furthermore, 44.8 and 30.4% of ARCs were associated with plasmids and MGEs, respectively. ARCs carrying genes for resistance to β-lactams, chloramphenicol, and tetracyclines were frequently identified as mobile, indicating a high potential for horizontal gene transfer and suggesting possible antibiotic contamination in the lake. Overall, this study provides the first metagenomic insight into the resistome of Laguna Lake using short-read sequencing and highlights its role as an environmental reservoir of mobile ARGs. The findings underscore the need for expanded ARG surveillance to improve antimicrobial resistance risk prediction.},
}
RevDate: 2026-02-09
CmpDate: 2026-02-09
Antibiotic Resistance of Escherichia coli and Klebsiella pneumoniae Isolated from Wild Raccoon Dogs in Shanghai, China.
Microbial drug resistance (Larchmont, N.Y.), 32(1):9-23.
Wild animals may act as reservoirs for resistant bacteria, and resident bacteria carried by wild animals in cities may also be subject to anthropogenic pressures that affect their resistance. This study aimed to evaluate the antibiotic susceptibility and biofilm formation ability of Escherichia coli and Klebsiella pneumoniae isolated from wild raccoon dogs from Shanghai, China, and to identify the genes responsible for resistance to different classes of antibiotics. The horizontal transfer of resistant plasmids was assessed by plasmid conjugation assays and characterized by third-generation nanopore sequencing. E. coli and K. pneumoniae isolated from wild raccoon dogs in Shanghai had strong biofilm formation ability. They had high resistance rates to amoxicillin, co-trimoxazole, tetracycline, and other antibiotics, but they were still sensitive to advanced antibiotics. The isolates contained prevalent resistance genes and virulence genes, and plasmids could be transferred horizontally. The resistant plasmids are rich in gene transfer elements such as insertion sequences. This is the first description of the antimicrobial resistance status and genes of wild raccoon dogs in Shanghai. These results highlight the urgent need to understand the origin and spread of resistance genes in wild animals such as urban raccoon dogs in Shanghai.
Additional Links: PMID-41182825
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41182825,
year = {2026},
author = {Xiong, X and Chen, C and Tao, J and Wei, M and Wang, X and Wang, C and Ye, W and Zhou, W and Liu, G and Zhang, K},
title = {Antibiotic Resistance of Escherichia coli and Klebsiella pneumoniae Isolated from Wild Raccoon Dogs in Shanghai, China.},
journal = {Microbial drug resistance (Larchmont, N.Y.)},
volume = {32},
number = {1},
pages = {9-23},
doi = {10.1177/10766294251392571},
pmid = {41182825},
issn = {1931-8448},
mesh = {Animals ; *Klebsiella pneumoniae/drug effects/genetics/isolation & purification ; *Escherichia coli/drug effects/genetics/isolation & purification ; China ; *Anti-Bacterial Agents/pharmacology ; *Raccoon Dogs/microbiology ; Plasmids/genetics ; Microbial Sensitivity Tests ; Gene Transfer, Horizontal ; *Drug Resistance, Multiple, Bacterial/genetics ; Animals, Wild/microbiology ; Biofilms/drug effects/growth & development ; },
abstract = {Wild animals may act as reservoirs for resistant bacteria, and resident bacteria carried by wild animals in cities may also be subject to anthropogenic pressures that affect their resistance. This study aimed to evaluate the antibiotic susceptibility and biofilm formation ability of Escherichia coli and Klebsiella pneumoniae isolated from wild raccoon dogs from Shanghai, China, and to identify the genes responsible for resistance to different classes of antibiotics. The horizontal transfer of resistant plasmids was assessed by plasmid conjugation assays and characterized by third-generation nanopore sequencing. E. coli and K. pneumoniae isolated from wild raccoon dogs in Shanghai had strong biofilm formation ability. They had high resistance rates to amoxicillin, co-trimoxazole, tetracycline, and other antibiotics, but they were still sensitive to advanced antibiotics. The isolates contained prevalent resistance genes and virulence genes, and plasmids could be transferred horizontally. The resistant plasmids are rich in gene transfer elements such as insertion sequences. This is the first description of the antimicrobial resistance status and genes of wild raccoon dogs in Shanghai. These results highlight the urgent need to understand the origin and spread of resistance genes in wild animals such as urban raccoon dogs in Shanghai.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
Animals
*Klebsiella pneumoniae/drug effects/genetics/isolation & purification
*Escherichia coli/drug effects/genetics/isolation & purification
China
*Anti-Bacterial Agents/pharmacology
*Raccoon Dogs/microbiology
Plasmids/genetics
Microbial Sensitivity Tests
Gene Transfer, Horizontal
*Drug Resistance, Multiple, Bacterial/genetics
Animals, Wild/microbiology
Biofilms/drug effects/growth & development
RevDate: 2026-02-07
Outbreak investigation and genomic analysis reveal hidden transmission networks of KPC-2-producing Enterobacterales in a South Korean hospital.
Antimicrobial resistance and infection control pii:10.1186/s13756-026-01706-x [Epub ahead of print].
BACKGROUND: We investigated a KPC-2-producing Enterobacterales (KPC-2 CPE) outbreak in a Korean hospital from July to September 2019, which subsided following enhanced surveillance and strict infection control. The study aimed to elucidate transmission dynamics using epidemiological and genomic methods.
METHODS: The study period covered the outbreak and a 9-month post-outbreak observation. Investigations included a matched case-control study and whole-genome sequencing (WGS) of isolates, including long-read sequencing for two isolates. Single nucleotide polymorphism (SNP) analysis (≤ 6 SNPs for clonality, ≤ 15 for relatedness) was used to construct transmission networks.
RESULTS: A total of 42 KPC-2 CPE cases were identified: 34 Klebsiella pneumoniae, 4 Escherichia coli, 1 Enterobacter asburiae, and 3 cases co-colonized with K. pneumoniae and E. coli. Among these, 33 were hospital-linked and 9 were imported. Retrospective tracing indicated that covert transmission began a month before the outbreak, and 13 hospital wards were identified as potential acquisition sites. Genomic analysis revealed all but one K. pneumoniae belonged to ST307, cgMLST 439, which grouped into three clades. Clade 1 was linked to a specific hospital ward, supported by the case-control study (adjusted odds ratio, 3.63; 95% confidence interval, 1.36-9.63); Clade 2 was spread between wards via a haemodialysis unit and shared healthcare personnel. Imported cases had the same clones as early hospital-linked cases, suggesting undetected introduction before enhanced surveillance. Additionally, an IncX3 plasmid carrying blaKPC-2 was found in both K. pneumoniae and E. coli, indicating horizontal gene transfer.
CONCLUSION: This study demonstrates that clonal spread of KPC-2 CPE can remain undetected without enhanced active surveillance, underscoring the need for early detection. Genomic analysis clarified ST307 K. pneumoniae transmission through unrecognised epidemiological links and horizontal blaKPC-2 transfer to E. coli.
Additional Links: PMID-41654982
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41654982,
year = {2026},
author = {Park, SH and Ji, SK and Shin, S and Park, C and Shin, JI and Jung, SH and Choi, JH and Lee, DG},
title = {Outbreak investigation and genomic analysis reveal hidden transmission networks of KPC-2-producing Enterobacterales in a South Korean hospital.},
journal = {Antimicrobial resistance and infection control},
volume = {},
number = {},
pages = {},
doi = {10.1186/s13756-026-01706-x},
pmid = {41654982},
issn = {2047-2994},
support = {2021R1A2C1009867//National Research Foundation of Korea/ ; },
abstract = {BACKGROUND: We investigated a KPC-2-producing Enterobacterales (KPC-2 CPE) outbreak in a Korean hospital from July to September 2019, which subsided following enhanced surveillance and strict infection control. The study aimed to elucidate transmission dynamics using epidemiological and genomic methods.
METHODS: The study period covered the outbreak and a 9-month post-outbreak observation. Investigations included a matched case-control study and whole-genome sequencing (WGS) of isolates, including long-read sequencing for two isolates. Single nucleotide polymorphism (SNP) analysis (≤ 6 SNPs for clonality, ≤ 15 for relatedness) was used to construct transmission networks.
RESULTS: A total of 42 KPC-2 CPE cases were identified: 34 Klebsiella pneumoniae, 4 Escherichia coli, 1 Enterobacter asburiae, and 3 cases co-colonized with K. pneumoniae and E. coli. Among these, 33 were hospital-linked and 9 were imported. Retrospective tracing indicated that covert transmission began a month before the outbreak, and 13 hospital wards were identified as potential acquisition sites. Genomic analysis revealed all but one K. pneumoniae belonged to ST307, cgMLST 439, which grouped into three clades. Clade 1 was linked to a specific hospital ward, supported by the case-control study (adjusted odds ratio, 3.63; 95% confidence interval, 1.36-9.63); Clade 2 was spread between wards via a haemodialysis unit and shared healthcare personnel. Imported cases had the same clones as early hospital-linked cases, suggesting undetected introduction before enhanced surveillance. Additionally, an IncX3 plasmid carrying blaKPC-2 was found in both K. pneumoniae and E. coli, indicating horizontal gene transfer.
CONCLUSION: This study demonstrates that clonal spread of KPC-2 CPE can remain undetected without enhanced active surveillance, underscoring the need for early detection. Genomic analysis clarified ST307 K. pneumoniae transmission through unrecognised epidemiological links and horizontal blaKPC-2 transfer to E. coli.},
}
RevDate: 2026-02-06
Micro- and nanoplastics facilitate the propagation of antimicrobial resistance in mixed microbial consortia.
Cell reports, 45(2):116946 pii:S2211-1247(26)00024-0 [Epub ahead of print].
Navigating the emerging pollutant crisis appears increasingly daunting, with the interaction between micro- and nanoplastics (M/NPs) and antimicrobial resistance (AMR) in complex microbial consortia remaining poorly understood. Here, mixed-culture microcosms are subjected to polymer- and size-resolved plastic exposures, and resistome and mobilome dynamics are quantified using phenotyping and multi-omics. M/NP exposure increases AMR gene abundance and reshapes resistance profiles in a polymer-dependent manner, dominated by efflux and target alteration. Particle miniaturization amplifies resistome diversity and gene mobility, and nanoplastics show the highest horizontal gene transfer activity and strongest co-localization of AMR genes with mobile genetic elements, forming dense cross-phylum transfer networks. Mechanistically, nanoplastics elevate ROS and membrane damage, activate the SOS response, and upregulate conjugation, competence, and transposase functions. Increased ATP generation and efflux activity sustain stress tolerance and energy-intensive DNA exchange, turning nanoplastics into hotspots of transferable resistance with implications for microbial evolution and ecological resilience.
Additional Links: PMID-41649927
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41649927,
year = {2026},
author = {Zhen, J and Wei, W and Duan, H and Hou, Y and Chen, X and Liu, Y and Ni, SQ and Ni, BJ},
title = {Micro- and nanoplastics facilitate the propagation of antimicrobial resistance in mixed microbial consortia.},
journal = {Cell reports},
volume = {45},
number = {2},
pages = {116946},
doi = {10.1016/j.celrep.2026.116946},
pmid = {41649927},
issn = {2211-1247},
abstract = {Navigating the emerging pollutant crisis appears increasingly daunting, with the interaction between micro- and nanoplastics (M/NPs) and antimicrobial resistance (AMR) in complex microbial consortia remaining poorly understood. Here, mixed-culture microcosms are subjected to polymer- and size-resolved plastic exposures, and resistome and mobilome dynamics are quantified using phenotyping and multi-omics. M/NP exposure increases AMR gene abundance and reshapes resistance profiles in a polymer-dependent manner, dominated by efflux and target alteration. Particle miniaturization amplifies resistome diversity and gene mobility, and nanoplastics show the highest horizontal gene transfer activity and strongest co-localization of AMR genes with mobile genetic elements, forming dense cross-phylum transfer networks. Mechanistically, nanoplastics elevate ROS and membrane damage, activate the SOS response, and upregulate conjugation, competence, and transposase functions. Increased ATP generation and efflux activity sustain stress tolerance and energy-intensive DNA exchange, turning nanoplastics into hotspots of transferable resistance with implications for microbial evolution and ecological resilience.},
}
RevDate: 2026-02-06
CmpDate: 2026-02-06
Molecular mimicry and trafficking of peptide effectors in sedentary nematodes: emerging drivers of feeding site formation and host signaling hijack.
Crop health, 4(1):1.
Despite significant advances in understanding the biology of plant-parasitic nematodes, the emergence of peptide hormone mimicry as a virulence strategy presents a complex facet of nematode parasitism. This review integrates recent advances on how nematode effectors, such as CLEs, CEPs, RALFs, IDA, and PSYs, are processed, post-translationally modified, and trafficked to hijack host signaling and developmental programs. By linking structural mimicry with receptor engagement and subcellular targeting, we highlight how these effectors reprogram plant transcriptional and immune responses to drive the formation of nematode feeding sites. We further explore the evolutionary origins of these effectors, emphasizing how processes such as horizontal gene transfer, neofunctionalization, and convergent selection have shaped peptide effectors into lineage-specific virulence factors. Finally, we outline critical research gaps focusing on structural and computational analyses of effector-receptor interfaces, functional genomics of trafficking and activation and translational opportunities for engineering durable host resistance. Together, these insights underscore the influence of molecular mimicry on nematode virulence and position effector biology as a frontier for translational innovation in crop protection.
Additional Links: PMID-41649717
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41649717,
year = {2026},
author = {Yusuf, AG and Bello, TT and Anifiwoshe, SO},
title = {Molecular mimicry and trafficking of peptide effectors in sedentary nematodes: emerging drivers of feeding site formation and host signaling hijack.},
journal = {Crop health},
volume = {4},
number = {1},
pages = {1},
pmid = {41649717},
issn = {2948-1945},
abstract = {Despite significant advances in understanding the biology of plant-parasitic nematodes, the emergence of peptide hormone mimicry as a virulence strategy presents a complex facet of nematode parasitism. This review integrates recent advances on how nematode effectors, such as CLEs, CEPs, RALFs, IDA, and PSYs, are processed, post-translationally modified, and trafficked to hijack host signaling and developmental programs. By linking structural mimicry with receptor engagement and subcellular targeting, we highlight how these effectors reprogram plant transcriptional and immune responses to drive the formation of nematode feeding sites. We further explore the evolutionary origins of these effectors, emphasizing how processes such as horizontal gene transfer, neofunctionalization, and convergent selection have shaped peptide effectors into lineage-specific virulence factors. Finally, we outline critical research gaps focusing on structural and computational analyses of effector-receptor interfaces, functional genomics of trafficking and activation and translational opportunities for engineering durable host resistance. Together, these insights underscore the influence of molecular mimicry on nematode virulence and position effector biology as a frontier for translational innovation in crop protection.},
}
RevDate: 2026-02-06
Assessment of genome evolution in Bifidobacterium adolescentis indicates genetic adaptation to the human gut.
mSystems [Epub ahead of print].
UNLABELLED: Bifidobacterium adolescentis is one of the most frequently encountered bifidobacterial species present in the adult human gut microbiota, with a prevalence of approximately 60%. Despite its high prevalence, B. adolescentis has not been extensively studied and characterized, and our understanding of its physiological traits, genetic diversity, and potential interactions with other members of the human gut microbiota or with its host is therefore fragmentary. In the current study, a data set comprising 1,682 B. adolescentis genomes was compiled by combining publicly available data and metagenome assemblies from 131 projects to uncover the unique genetic characteristics of this species. A pangenome analysis of B. adolescentis identified 203 clusters of orthologous genes absent from the other five human-associated Bifidobacterium species, six of which were in silico predicted to encode functions unique to this taxon. Furthermore, 2,597 genes were predicted to have been acquired by horizontal gene transfer, including genes encoding extracellular structures involved in interaction with the host and other microorganisms, and phage defense mechanisms against bacteriophages. Detailed phylogenetic analysis revealed seven clusters within the B. adolescentis species, each partially associated with the origin of strain isolation, suggesting phylogenetic differentiation shaped by geographical strain origin. Moreover, a large-scale metagenomic analysis of over 10,000 human gut metagenomes from healthy adults revealed that B. adolescentis co-occurs with 36 putative beneficial commensals and butyrate-producing taxa, highlighting its role as a key bifidobacterial species involved in microbial networking within the adult human gut microbiota.
IMPORTANCE: To comprehensively explore the biodiversity within a microbial species, the reconstruction of a substantial number of genomes is essential. In this study, we successfully uncovered the genetic diversity of Bifidobacterium adolescentis by retrieving a large number of genomes from human gut metagenomic samples. The complete overview of the B. adolescentis pangenome enabled us to investigate the genetic features that distinguish this gut commensal from other bifidobacterial species residing in the human intestinal microbiota.
Additional Links: PMID-41649278
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41649278,
year = {2026},
author = {Selleri, E and Tarracchini, C and Petraro, S and Mancabelli, L and Milani, C and Turroni, F and Shao, Y and Browne, HP and Lawley, TD and van Sinderen, D and Ventura, M and Lugli, GA},
title = {Assessment of genome evolution in Bifidobacterium adolescentis indicates genetic adaptation to the human gut.},
journal = {mSystems},
volume = {},
number = {},
pages = {e0117325},
doi = {10.1128/msystems.01173-25},
pmid = {41649278},
issn = {2379-5077},
abstract = {UNLABELLED: Bifidobacterium adolescentis is one of the most frequently encountered bifidobacterial species present in the adult human gut microbiota, with a prevalence of approximately 60%. Despite its high prevalence, B. adolescentis has not been extensively studied and characterized, and our understanding of its physiological traits, genetic diversity, and potential interactions with other members of the human gut microbiota or with its host is therefore fragmentary. In the current study, a data set comprising 1,682 B. adolescentis genomes was compiled by combining publicly available data and metagenome assemblies from 131 projects to uncover the unique genetic characteristics of this species. A pangenome analysis of B. adolescentis identified 203 clusters of orthologous genes absent from the other five human-associated Bifidobacterium species, six of which were in silico predicted to encode functions unique to this taxon. Furthermore, 2,597 genes were predicted to have been acquired by horizontal gene transfer, including genes encoding extracellular structures involved in interaction with the host and other microorganisms, and phage defense mechanisms against bacteriophages. Detailed phylogenetic analysis revealed seven clusters within the B. adolescentis species, each partially associated with the origin of strain isolation, suggesting phylogenetic differentiation shaped by geographical strain origin. Moreover, a large-scale metagenomic analysis of over 10,000 human gut metagenomes from healthy adults revealed that B. adolescentis co-occurs with 36 putative beneficial commensals and butyrate-producing taxa, highlighting its role as a key bifidobacterial species involved in microbial networking within the adult human gut microbiota.
IMPORTANCE: To comprehensively explore the biodiversity within a microbial species, the reconstruction of a substantial number of genomes is essential. In this study, we successfully uncovered the genetic diversity of Bifidobacterium adolescentis by retrieving a large number of genomes from human gut metagenomic samples. The complete overview of the B. adolescentis pangenome enabled us to investigate the genetic features that distinguish this gut commensal from other bifidobacterial species residing in the human intestinal microbiota.},
}
RevDate: 2026-02-06
Synthetic overlapping genes stabilize genetic systems.
mBio [Epub ahead of print].
UNLABELLED: Overlapping genes-wherein two different proteins are translated from alternative reading frames of the same DNA sequence-provide a means to stabilize an engineered gene by directly linking its evolutionary fate with that of an overlapping gene. However, creating overlapping gene pairs is challenging, as it requires redesigning both protein products to accommodate overlap constraints. Here, we present a new "overlapping, alternate-frame insertion" (OAFI) method for creating synthetic overlapping genes by inserting an "inner" gene, encoded in an alternate frame, into a flexible region of an "outer" gene. Using OAFI, we create new overlapping gene pairs of genetic reporters and bacterial toxins within an antibiotic resistance gene. We show that both the inner and outer genes retain function despite redesign, with translation of the inner gene influenced by its overlap position in the outer gene. Importantly, we show that, despite these inner gene sequences not contributing to outer gene function, selection for the outer gene alters the permitted inactivating mutations in the inner gene, and that overlapping toxins can restrict horizontal gene transfer of the antibiotic resistance gene. Overall, OAFI offers a versatile tool for synthetic biology, expanding the applications of overlapping genes in gene stabilization and biocontainment.
IMPORTANCE: Genetically engineered microbes promise to improve human health and help solve global climate crises. However, the widespread adoption of these microbes is often hindered by genetic instability caused by mutations and by the unpredictable spread of synthetic genes in the environment. We present a simple but effective method for creating synthetic overlapping genes to stabilize genes against mutations and prevent their spread in the environment. This method is broadly useful for constructing stable genetically engineered microbes and studying how they evolve in the environment.
Additional Links: PMID-41649272
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41649272,
year = {2026},
author = {Leonard, SP and Halvorsen, TM and Lim, B and McCall, NA and Park, DM and Jiao, Y and Yung, MC and Ricci, DP},
title = {Synthetic overlapping genes stabilize genetic systems.},
journal = {mBio},
volume = {},
number = {},
pages = {e0272525},
doi = {10.1128/mbio.02725-25},
pmid = {41649272},
issn = {2150-7511},
abstract = {UNLABELLED: Overlapping genes-wherein two different proteins are translated from alternative reading frames of the same DNA sequence-provide a means to stabilize an engineered gene by directly linking its evolutionary fate with that of an overlapping gene. However, creating overlapping gene pairs is challenging, as it requires redesigning both protein products to accommodate overlap constraints. Here, we present a new "overlapping, alternate-frame insertion" (OAFI) method for creating synthetic overlapping genes by inserting an "inner" gene, encoded in an alternate frame, into a flexible region of an "outer" gene. Using OAFI, we create new overlapping gene pairs of genetic reporters and bacterial toxins within an antibiotic resistance gene. We show that both the inner and outer genes retain function despite redesign, with translation of the inner gene influenced by its overlap position in the outer gene. Importantly, we show that, despite these inner gene sequences not contributing to outer gene function, selection for the outer gene alters the permitted inactivating mutations in the inner gene, and that overlapping toxins can restrict horizontal gene transfer of the antibiotic resistance gene. Overall, OAFI offers a versatile tool for synthetic biology, expanding the applications of overlapping genes in gene stabilization and biocontainment.
IMPORTANCE: Genetically engineered microbes promise to improve human health and help solve global climate crises. However, the widespread adoption of these microbes is often hindered by genetic instability caused by mutations and by the unpredictable spread of synthetic genes in the environment. We present a simple but effective method for creating synthetic overlapping genes to stabilize genes against mutations and prevent their spread in the environment. This method is broadly useful for constructing stable genetically engineered microbes and studying how they evolve in the environment.},
}
RevDate: 2026-02-06
CmpDate: 2026-02-06
Type IV Secretion System Drives Lipid Mixing.
bioRxiv : the preprint server for biology pii:2026.01.14.699567.
UNLABELLED: Type IV secretion systems (T4SSs) are versatile molecular machines used by bacteria to secrete protein effectors into host cells, promoting pathogenesis, and to transfer DNA between bacteria through conjugation, driving horizontal gene transfer. Most, like Dot/Icm of the pathogen Legionella pneumophila (L. pneumophila) or Escherichia coli (E. coli) RK2, are primed for substrate delivery only upon contact with a target membrane, but mechanisms are unknown. A pilus could bind a receptor to initiate priming, but many T4SSs, especially those that deliver effectors, lack a pilus. Here, we present evidence that T4SSs are primed by direct contact with target membrane lipids. Combining fluorescence assays with genetics and biochemistry, we found that Dot/Icm drives lipid exchange between bacterial cells and between bacteria and synthetic membranes containing only lipids. Lipid exchange requires membrane contact but does not require ATP hydrolysis or even full complex assembly. Minimally, the outer membrane core complex protein DotG needs to be present in at least one of the apposed membranes. We similarly observed lipid mixing with the simpler E. coli RK2 T4SS, where we could follow lipid mixing and plasmid transfer simultaneously. We found that lipid mixing always preceded or accompanied plasmid transfer, suggesting it may be part of the contact-dependent priming mechanism. Lipid mixing was inhibited or promoted by lipids that inhibit or promote membrane fusion, respectively. Lipids inhibiting lipid mixing also inhibited substrate transfer. Together, our results suggest that initial contact between DotG outer segments and target membrane lipids promotes lipid mixing as part of the mechanism that primes T4SS for substrate translocation.
HIGHLIGHTS: The Legionella pneumophila Dot/Icm secretion system requires contact with a target membrane for effector translocation, but how membrane contact primes the machinery for this is unknown. We found that Dot/Icm drives contact-dependent lipid mixing between bacteria or between bacteria and liposomes, showing that Dot/Icm priming does not require a protein receptor. Lipid mixing also occurs with the simpler Type IVA system (Escherichia coli RK2) during conjugation, suggesting lipid mixing is a general feature of Type IV Secretion System function. The core complex protein DotG is sufficient to drive lipid mixing, suggesting DotG-target membrane interactions may destabilize the target membrane.
Additional Links: PMID-41648637
Full Text:
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41648637,
year = {2026},
author = {Chetrit, D and Roy, CR and Karatekin, E},
title = {Type IV Secretion System Drives Lipid Mixing.},
journal = {bioRxiv : the preprint server for biology},
volume = {},
number = {},
pages = {},
doi = {10.64898/2026.01.14.699567},
pmid = {41648637},
issn = {2692-8205},
abstract = {UNLABELLED: Type IV secretion systems (T4SSs) are versatile molecular machines used by bacteria to secrete protein effectors into host cells, promoting pathogenesis, and to transfer DNA between bacteria through conjugation, driving horizontal gene transfer. Most, like Dot/Icm of the pathogen Legionella pneumophila (L. pneumophila) or Escherichia coli (E. coli) RK2, are primed for substrate delivery only upon contact with a target membrane, but mechanisms are unknown. A pilus could bind a receptor to initiate priming, but many T4SSs, especially those that deliver effectors, lack a pilus. Here, we present evidence that T4SSs are primed by direct contact with target membrane lipids. Combining fluorescence assays with genetics and biochemistry, we found that Dot/Icm drives lipid exchange between bacterial cells and between bacteria and synthetic membranes containing only lipids. Lipid exchange requires membrane contact but does not require ATP hydrolysis or even full complex assembly. Minimally, the outer membrane core complex protein DotG needs to be present in at least one of the apposed membranes. We similarly observed lipid mixing with the simpler E. coli RK2 T4SS, where we could follow lipid mixing and plasmid transfer simultaneously. We found that lipid mixing always preceded or accompanied plasmid transfer, suggesting it may be part of the contact-dependent priming mechanism. Lipid mixing was inhibited or promoted by lipids that inhibit or promote membrane fusion, respectively. Lipids inhibiting lipid mixing also inhibited substrate transfer. Together, our results suggest that initial contact between DotG outer segments and target membrane lipids promotes lipid mixing as part of the mechanism that primes T4SS for substrate translocation.
HIGHLIGHTS: The Legionella pneumophila Dot/Icm secretion system requires contact with a target membrane for effector translocation, but how membrane contact primes the machinery for this is unknown. We found that Dot/Icm drives contact-dependent lipid mixing between bacteria or between bacteria and liposomes, showing that Dot/Icm priming does not require a protein receptor. Lipid mixing also occurs with the simpler Type IVA system (Escherichia coli RK2) during conjugation, suggesting lipid mixing is a general feature of Type IV Secretion System function. The core complex protein DotG is sufficient to drive lipid mixing, suggesting DotG-target membrane interactions may destabilize the target membrane.},
}
RevDate: 2026-02-06
CmpDate: 2026-02-06
Low affinity DNA-binding promotes cooperative activation of natural transformation in Vibrio cholerae.
bioRxiv : the preprint server for biology pii:2026.01.21.700895.
UNLABELLED: DNA-binding transcriptional regulators control gene expression in response to environmental cues. A subset of these proteins, called transmembrane transcriptional regulators (TTRs), directly bind DNA to regulate transcription while remaining anchored in the cytoplasmic membrane. Prior work has shown that in the presence of the polysaccharide chitin, two TTRs, TfoS and ChiS, coordinate to induce the expression of TfoR, a small RNA that is critical for natural transformation in Vibrio cholerae . Specifically, it was shown that ChiS recruits the P tfoR locus to the membrane, which allows for the subsequent activation of this promoter by TfoS. However, it was also shown that increasing TfoS protein levels bypasses this coordination, allowing TfoS to activate the promoter independently. It therefore remains unclear what molecular mechanisms drive the requirement for ChiS in native conditions. Here, we show that ChiS binds P tfoR with a higher affinity than TfoS. We hypothesized that the low affinity of TfoS for P tfoR helps reinforce its dependence on ChiS for activation. To test this, we isolated a mutant allele of the TfoS DNA-binding domain that has a higher affinity for P tfoR . We show that this high-affinity TfoS allele promotes ChiS-independent activation of P tfoR . These results demonstrate that the relative DNA-binding affinity of TTRs is a critical feature that drives their coordination.
IMPORTANCE: DNA-binding transmembrane transcriptional regulators (TTRs) are critical for some bacterial species to properly sense and respond to their environments. Recent work highlights that pairs of TTRs can coordinate their activities to regulate gene expression, allowing them to sensitively control behaviors like virulence and horizontal gene transfer. However, the mechanisms that enable this coordination remain poorly understood. Here, we show that the relative DNA-binding affinity of paired TTRs is a critical feature that can drive their coordination.
Additional Links: PMID-41648574
Full Text:
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41648574,
year = {2026},
author = {Hullinger, AC and Callahan, VE and Dalia, AB},
title = {Low affinity DNA-binding promotes cooperative activation of natural transformation in Vibrio cholerae.},
journal = {bioRxiv : the preprint server for biology},
volume = {},
number = {},
pages = {},
doi = {10.64898/2026.01.21.700895},
pmid = {41648574},
issn = {2692-8205},
abstract = {UNLABELLED: DNA-binding transcriptional regulators control gene expression in response to environmental cues. A subset of these proteins, called transmembrane transcriptional regulators (TTRs), directly bind DNA to regulate transcription while remaining anchored in the cytoplasmic membrane. Prior work has shown that in the presence of the polysaccharide chitin, two TTRs, TfoS and ChiS, coordinate to induce the expression of TfoR, a small RNA that is critical for natural transformation in Vibrio cholerae . Specifically, it was shown that ChiS recruits the P tfoR locus to the membrane, which allows for the subsequent activation of this promoter by TfoS. However, it was also shown that increasing TfoS protein levels bypasses this coordination, allowing TfoS to activate the promoter independently. It therefore remains unclear what molecular mechanisms drive the requirement for ChiS in native conditions. Here, we show that ChiS binds P tfoR with a higher affinity than TfoS. We hypothesized that the low affinity of TfoS for P tfoR helps reinforce its dependence on ChiS for activation. To test this, we isolated a mutant allele of the TfoS DNA-binding domain that has a higher affinity for P tfoR . We show that this high-affinity TfoS allele promotes ChiS-independent activation of P tfoR . These results demonstrate that the relative DNA-binding affinity of TTRs is a critical feature that drives their coordination.
IMPORTANCE: DNA-binding transmembrane transcriptional regulators (TTRs) are critical for some bacterial species to properly sense and respond to their environments. Recent work highlights that pairs of TTRs can coordinate their activities to regulate gene expression, allowing them to sensitively control behaviors like virulence and horizontal gene transfer. However, the mechanisms that enable this coordination remain poorly understood. Here, we show that the relative DNA-binding affinity of paired TTRs is a critical feature that can drive their coordination.},
}
RevDate: 2026-02-06
CmpDate: 2026-02-06
An activity-resistance tradeoff constrains enzyme evolution.
bioRxiv : the preprint server for biology pii:2026.01.19.700455.
UNLABELLED: The presence of self-resistance genes in antibiotic-producing organisms poses a paradox: how can resistance evolve before the antibiotic exists, and how can an antibiotic producer arise without first evolving resistance? Here we examine the evolutionary origins of self-resistance to mycophenolic acid (MPA), an inhibitor of inosine monophosphate dehydrogenase (IMPDH). The MPA biosynthetic gene cluster (BGC) includes a resistant IMPDH-B. Homologs of IMPDH-B occur not only in MPA producers but also in many non-producing fungi, where remnants of the MPA BGC remain detectable. The phylogeny of IMPDH-B is incongruent with the fungal species tree, consistent with multiple horizontal gene transfer events between Aspergillus and Sordariomycetes. We characterized eleven extant IMPDH-Bs, five from MPA producers and six from nonproducers, along with seven resurrected ancestral enzymes (Anc1-Anc7). MPA resistance appeared between Anc2 and Anc3 and coincided with a loss of catalytic efficiency. Across both ancestral and extant enzymes, MPA resistance correlated strongly with reduced activity, revealing a robust activity-resistance trade-off that has persisted for millions of years. Unexpectedly, both the IMPDH-Bs and ancestral enzymes Anc3-Anc7 were also resistant to ribavirin-5'-monophosphate (RVP), an IMP-competitive inhibitor. Because MPA and RVP bind to similar enzyme conformations, the activity-resistance trade-off likely reflects a design constraint imposed by the need to maintain resistance to multiple inhibitors. Intriguingly, although Anc1 and Anc2 are equally sensitive to MPA, Anc2 shows reduced susceptibility to RVP. This pattern suggests that pre-existing resistance to another IMPDH inhibitor may have created a permissive background for the later evolution of MPA biosynthesis.
SIGNIFICANCE: Antibiotic producers must be resistant to the toxins that they produce, but how such self-resistance develops is a mystery. The mycophenolic acid (MPA) biosynthetic gene cluster (BGC) encodes a resistant variant of the MPA target IMPDH (IMPDH-B). Many fungi retain IMPDH-B although they have lost the ability to produce MPA. The IMPDH-B and species phylogenies are incongruent, suggesting evolution of the BGC was complicated. MPA resistance correlates with low catalytic efficiency in modern and ancestral IMPDHs, revealing a robust design constraint tradeoff. Surprisingly, IMPDH-Bs are also resistant to an IMP-competitive inhibitor (RVP). RVP resistance appears to have emerged before MPA resistance. Perhaps resistance to RVP created a background that permitted the genesis of a new toxin.
Additional Links: PMID-41648278
Full Text:
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41648278,
year = {2026},
author = {Sarkis, AW and Sørensen, JL and Sondergaard, TE and Nielsen, KL and Frisvad, JC and Theobald, DL and Hedstrom, L},
title = {An activity-resistance tradeoff constrains enzyme evolution.},
journal = {bioRxiv : the preprint server for biology},
volume = {},
number = {},
pages = {},
doi = {10.64898/2026.01.19.700455},
pmid = {41648278},
issn = {2692-8205},
abstract = {UNLABELLED: The presence of self-resistance genes in antibiotic-producing organisms poses a paradox: how can resistance evolve before the antibiotic exists, and how can an antibiotic producer arise without first evolving resistance? Here we examine the evolutionary origins of self-resistance to mycophenolic acid (MPA), an inhibitor of inosine monophosphate dehydrogenase (IMPDH). The MPA biosynthetic gene cluster (BGC) includes a resistant IMPDH-B. Homologs of IMPDH-B occur not only in MPA producers but also in many non-producing fungi, where remnants of the MPA BGC remain detectable. The phylogeny of IMPDH-B is incongruent with the fungal species tree, consistent with multiple horizontal gene transfer events between Aspergillus and Sordariomycetes. We characterized eleven extant IMPDH-Bs, five from MPA producers and six from nonproducers, along with seven resurrected ancestral enzymes (Anc1-Anc7). MPA resistance appeared between Anc2 and Anc3 and coincided with a loss of catalytic efficiency. Across both ancestral and extant enzymes, MPA resistance correlated strongly with reduced activity, revealing a robust activity-resistance trade-off that has persisted for millions of years. Unexpectedly, both the IMPDH-Bs and ancestral enzymes Anc3-Anc7 were also resistant to ribavirin-5'-monophosphate (RVP), an IMP-competitive inhibitor. Because MPA and RVP bind to similar enzyme conformations, the activity-resistance trade-off likely reflects a design constraint imposed by the need to maintain resistance to multiple inhibitors. Intriguingly, although Anc1 and Anc2 are equally sensitive to MPA, Anc2 shows reduced susceptibility to RVP. This pattern suggests that pre-existing resistance to another IMPDH inhibitor may have created a permissive background for the later evolution of MPA biosynthesis.
SIGNIFICANCE: Antibiotic producers must be resistant to the toxins that they produce, but how such self-resistance develops is a mystery. The mycophenolic acid (MPA) biosynthetic gene cluster (BGC) encodes a resistant variant of the MPA target IMPDH (IMPDH-B). Many fungi retain IMPDH-B although they have lost the ability to produce MPA. The IMPDH-B and species phylogenies are incongruent, suggesting evolution of the BGC was complicated. MPA resistance correlates with low catalytic efficiency in modern and ancestral IMPDHs, revealing a robust design constraint tradeoff. Surprisingly, IMPDH-Bs are also resistant to an IMP-competitive inhibitor (RVP). RVP resistance appears to have emerged before MPA resistance. Perhaps resistance to RVP created a background that permitted the genesis of a new toxin.},
}
RevDate: 2026-02-06
CmpDate: 2026-02-06
The stoichiometry of minor-to-major pilins regulates the dynamic activity of the type IVa competence pilus in Vibrio cholerae.
bioRxiv : the preprint server for biology pii:2026.01.17.700090.
UNLABELLED: Type IVa pili (T4aP) are bacterial surface appendages that perform various functions including twitching motility, surface attachment, cell-cell interactions, and DNA uptake for natural transformation. Pivotal to each of these functions is the ability of T4aP to be dynamically extended and retracted from the cell surface. However, the factors that regulate this dynamic activity remain poorly understood. To address this question, we employ the competence T4aP from Vibrio cholerae as a model system. T4aP are composed of major and minor pilin subunits, named based on their relative abundance in the pilus filament. Prior work has established that minor pilins form a complex that initiates T4aP assembly. This allows for the subsequent addition of major pilins to the filament, which promotes T4aP extension. Here, we uncover that the stoichiometry of minor-to-major pilins is a crucial determinant of T4aP dynamic activity. Specifically, we show that either (1) overexpressing minor pilins or (2) underexpressing the major pilin results is a dramatic increase in the frequency of T4aP dynamics. These results indicate that the stoichiometry of major-to-minor pilins, not their absolute abundance, is one mechanism that regulates T4aP dynamic activity.
AUTHOR SUMMARY: Type IVa pili (T4aP) are a broadly conserved family of filamentous bacterial appendages that help bacteria colonize surfaces, move towards or away from stimuli, and gain new traits through a mechanism of horizontal gene transfer called natural transformation. T4aP are primarily composed of protein subunits called major and minor pilins, named based on their relative abundance in the pilus filament. Bacteria can dynamically extend and retract pilus filaments from their surface through polymerization and depolymerization of these pilins. This dynamic activity is critical for the activities that T4aP carry out. However, the factors that regulate this dynamic activity remain incompletely understood. Here, we find that the ratio of minor-to-major pilins is one factor that regulates the frequency of dynamic activity. Minor pilins are a universally conserved feature of T4aP. So, the minor-to-major pilin ratio may be a broadly conserved mechanism for controlling dynamic T4aP activity in diverse bacterial species.
Additional Links: PMID-41648272
Full Text:
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41648272,
year = {2026},
author = {Christman, ND and Dalia, TN and Chlebek, JL and Dalia, AB},
title = {The stoichiometry of minor-to-major pilins regulates the dynamic activity of the type IVa competence pilus in Vibrio cholerae.},
journal = {bioRxiv : the preprint server for biology},
volume = {},
number = {},
pages = {},
doi = {10.64898/2026.01.17.700090},
pmid = {41648272},
issn = {2692-8205},
abstract = {UNLABELLED: Type IVa pili (T4aP) are bacterial surface appendages that perform various functions including twitching motility, surface attachment, cell-cell interactions, and DNA uptake for natural transformation. Pivotal to each of these functions is the ability of T4aP to be dynamically extended and retracted from the cell surface. However, the factors that regulate this dynamic activity remain poorly understood. To address this question, we employ the competence T4aP from Vibrio cholerae as a model system. T4aP are composed of major and minor pilin subunits, named based on their relative abundance in the pilus filament. Prior work has established that minor pilins form a complex that initiates T4aP assembly. This allows for the subsequent addition of major pilins to the filament, which promotes T4aP extension. Here, we uncover that the stoichiometry of minor-to-major pilins is a crucial determinant of T4aP dynamic activity. Specifically, we show that either (1) overexpressing minor pilins or (2) underexpressing the major pilin results is a dramatic increase in the frequency of T4aP dynamics. These results indicate that the stoichiometry of major-to-minor pilins, not their absolute abundance, is one mechanism that regulates T4aP dynamic activity.
AUTHOR SUMMARY: Type IVa pili (T4aP) are a broadly conserved family of filamentous bacterial appendages that help bacteria colonize surfaces, move towards or away from stimuli, and gain new traits through a mechanism of horizontal gene transfer called natural transformation. T4aP are primarily composed of protein subunits called major and minor pilins, named based on their relative abundance in the pilus filament. Bacteria can dynamically extend and retract pilus filaments from their surface through polymerization and depolymerization of these pilins. This dynamic activity is critical for the activities that T4aP carry out. However, the factors that regulate this dynamic activity remain incompletely understood. Here, we find that the ratio of minor-to-major pilins is one factor that regulates the frequency of dynamic activity. Minor pilins are a universally conserved feature of T4aP. So, the minor-to-major pilin ratio may be a broadly conserved mechanism for controlling dynamic T4aP activity in diverse bacterial species.},
}
RevDate: 2026-02-05
Integrated metagenomic and 16S rRNA analysis reveals temporal associations between resistance genes and microbial communities during dairy manure composting.
Scientific reports pii:10.1038/s41598-026-37092-y [Epub ahead of print].
Dairy manure composting is widely applied to stabilize organic waste and reduce environmental pollution, yet the behavior of resistance determinants during this process remains insufficiently resolved. In this study, shotgun metagenomic sequencing was used to characterize temporal changes in antibiotic resistance genes (ARGs), metal resistance genes (MRGs), biocide resistance genes (BRGs), mobile genetic elements (MGEs), and microbial community composition during dairy manure composting. Rather than inferring direct mechanistic causation, our analyses focused on identifying statistically supported trends, associations, and co-occurrence patterns across composting stages. We observed a rapid decline in the relative abundance of ARGs compared with MRGs and BRGs during the thermophilic phase, coinciding with increasing temperature, while specific genes such as sul2 persisted throughout the process. Shifts in microbial community composition, particularly changes in the relative dominance of Actinobacteria and Proteobacteria, were significantly associated with variations in resistome profiles. Correlation and network analyses further revealed strong associations among ARGs, MRGs, BRGs, and MGEs, suggesting potential co-selection and horizontal gene transfer linkages without implying direct causal mechanisms. In addition, several opportunistic bacterial genera showed positive associations with aminoglycoside- and macrolide-lincosamide-streptogramin-type ARGs, indicating possible dissemination risks following compost application. Overall, this study provides an integrated, association-based overview of resistome and microbial community dynamics during dairy manure composting and highlights the importance of considering multiple resistance determinants when evaluating composting as a manure management strategy.
Additional Links: PMID-41644585
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41644585,
year = {2026},
author = {Zhou, Y and Liu, K and Gong, P and Wu, J and Ren, Z and Jin, E},
title = {Integrated metagenomic and 16S rRNA analysis reveals temporal associations between resistance genes and microbial communities during dairy manure composting.},
journal = {Scientific reports},
volume = {},
number = {},
pages = {},
doi = {10.1038/s41598-026-37092-y},
pmid = {41644585},
issn = {2045-2322},
abstract = {Dairy manure composting is widely applied to stabilize organic waste and reduce environmental pollution, yet the behavior of resistance determinants during this process remains insufficiently resolved. In this study, shotgun metagenomic sequencing was used to characterize temporal changes in antibiotic resistance genes (ARGs), metal resistance genes (MRGs), biocide resistance genes (BRGs), mobile genetic elements (MGEs), and microbial community composition during dairy manure composting. Rather than inferring direct mechanistic causation, our analyses focused on identifying statistically supported trends, associations, and co-occurrence patterns across composting stages. We observed a rapid decline in the relative abundance of ARGs compared with MRGs and BRGs during the thermophilic phase, coinciding with increasing temperature, while specific genes such as sul2 persisted throughout the process. Shifts in microbial community composition, particularly changes in the relative dominance of Actinobacteria and Proteobacteria, were significantly associated with variations in resistome profiles. Correlation and network analyses further revealed strong associations among ARGs, MRGs, BRGs, and MGEs, suggesting potential co-selection and horizontal gene transfer linkages without implying direct causal mechanisms. In addition, several opportunistic bacterial genera showed positive associations with aminoglycoside- and macrolide-lincosamide-streptogramin-type ARGs, indicating possible dissemination risks following compost application. Overall, this study provides an integrated, association-based overview of resistome and microbial community dynamics during dairy manure composting and highlights the importance of considering multiple resistance determinants when evaluating composting as a manure management strategy.},
}
RevDate: 2026-02-05
Phylotranscriptomics reveals conflicts of deep nodes in Saxifragales.
Molecular phylogenetics and evolution pii:S1055-7903(26)00023-0 [Epub ahead of print].
Saxifragales comprises 15 families in five well-supported clades: Paeoniaceae, Peridiscaceae, the woody clade, Cynomoriaceae, and the core Saxifragales. Relationships among these groups-particularly the placements of Paeoniaceae and Cynomoriaceae, and family-level relationships within the woody clade-remain uncertain. Here, we analyzed transcriptomes from 88 species (13 families) and plastomes from 14 families (with limited plastid genes retained in the parasitic Cynomoriaceae). Phylogenomic analyses of 1,113 BUSCO single-copy nuclear genes and 78 plastid genes consistently recovered Paeoniaceae as sister to the woody clade (Paeoniaceae + Woody clade, PWC) and supported Cynomoriaceae as sister to the core Saxifragales (Cynomoriaceae + Core Saxifragales, CCS). We detected widespread phylogenetic conflict and cytonuclear discordance, largely driven by pervasive gene flow and, to a lesser extent, incomplete lineage sorting (ILS). Gene tree error contributed to the unstable placement of Cynomoriaceae, while ILS dominated conflicts involving Cercidiphyllaceae. Future work integrating chromosome-level genomes and karyotype evolution may clarify woody clade relationships, and account for horizontal gene transfer in Cynomoriaceae.
Additional Links: PMID-41643823
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41643823,
year = {2026},
author = {Li, HL and Chang, H and Xie, HH and Zhang, L and Hao, GQ and Dimitrov, D and Sun, PC and Walker-Hale, N and Li, JL and Xu, XT},
title = {Phylotranscriptomics reveals conflicts of deep nodes in Saxifragales.},
journal = {Molecular phylogenetics and evolution},
volume = {},
number = {},
pages = {108553},
doi = {10.1016/j.ympev.2026.108553},
pmid = {41643823},
issn = {1095-9513},
abstract = {Saxifragales comprises 15 families in five well-supported clades: Paeoniaceae, Peridiscaceae, the woody clade, Cynomoriaceae, and the core Saxifragales. Relationships among these groups-particularly the placements of Paeoniaceae and Cynomoriaceae, and family-level relationships within the woody clade-remain uncertain. Here, we analyzed transcriptomes from 88 species (13 families) and plastomes from 14 families (with limited plastid genes retained in the parasitic Cynomoriaceae). Phylogenomic analyses of 1,113 BUSCO single-copy nuclear genes and 78 plastid genes consistently recovered Paeoniaceae as sister to the woody clade (Paeoniaceae + Woody clade, PWC) and supported Cynomoriaceae as sister to the core Saxifragales (Cynomoriaceae + Core Saxifragales, CCS). We detected widespread phylogenetic conflict and cytonuclear discordance, largely driven by pervasive gene flow and, to a lesser extent, incomplete lineage sorting (ILS). Gene tree error contributed to the unstable placement of Cynomoriaceae, while ILS dominated conflicts involving Cercidiphyllaceae. Future work integrating chromosome-level genomes and karyotype evolution may clarify woody clade relationships, and account for horizontal gene transfer in Cynomoriaceae.},
}
RevDate: 2026-02-05
Cefiderocol-resistant Aeromonas with expanded Resistomes in German hospital wastewater: Phenotypic and genomic evidence from the environment-clinical Interface.
The Science of the total environment, 1017:181478 pii:S0048-9697(26)00138-5 [Epub ahead of print].
Hospital wastewater is a key interface between clinical and environmental reservoirs of antimicrobial resistance, fostering selection and horizontal gene transfer. Aeromonas spp. are aquatic opportunistic pathogens with highly plastic genomes and are increasingly recognized as potential intermediaries in resistance dissemination. We compared 72 cefiderocol-selected Aeromonas isolates recovered from untreated hospital wastewater collected at six tertiary care hospitals across Germany with 62 clinical isolates from patients with intestinal and extraintestinal infections, to characterize cefiderocol susceptibility, resistome composition, and genomic mobility features. Pangenome analysis revealed an open genome structure comprising 21,364 gene clusters, with a core genome of 2486 genes and a large cloud gene pool (15,612 clusters present in <15% of isolates), highlighting extensive genomic plasticity. Resistance phenotypes diverged markedly: cefiderocol-selected wastewater isolates exhibited high resistance rates to multiple clinically relevant agents - ciprofloxacin (93.1%), aztreonam (81.2%), and trimethoprim-sulfamethoxazole (38.9%), whereas clinical isolates remained largely susceptible overall (<10%). Under iron limitation, siderophore production increased in both cohorts; however, in the presence of cefiderocol it remained robust in wastewater isolates while being suppressed in clinical isolates. Comparative genomics showed that wastewater isolates carried substantially expanded resistomes (mean 13.8 ARGs; range 2-27) relative to clinical isolates (mean 2.6; range 1-11), including enrichment of clinically relevant β-lactamases and carbapenemases. This resistance burden coincided with a larger and more transmissible plasmidome and a high insertion sequence load. Notably, extensive plasmid-backbone homology was detected between Aeromonas and co-occurring cefiderocol-resistant Enterobacterales isolated from the same wastewater samples, highlighting interspecies gene flow at the hospital-environment interface. Together, these findings identify hospital wastewater as a reservoir and convergence point for highly resistant, mobilome-enriched Aeromonas subpopulations captured under cefiderocol selection, supporting Aeromonas as a One Health sentinel and emphasizing the value of wastewater-based surveillance for tracking mobile resistance determinants bridging environmental and clinical compartments.
Additional Links: PMID-41643596
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41643596,
year = {2026},
author = {Savin, M and Erler, T and Carlsen, L and Dengler, J and Hammerl, JA and Hoffmann, M and Knobloch, JK and Lübbert, C and Parcina, M and Schwanz, T and Zweigner, J and Mutters, NT},
title = {Cefiderocol-resistant Aeromonas with expanded Resistomes in German hospital wastewater: Phenotypic and genomic evidence from the environment-clinical Interface.},
journal = {The Science of the total environment},
volume = {1017},
number = {},
pages = {181478},
doi = {10.1016/j.scitotenv.2026.181478},
pmid = {41643596},
issn = {1879-1026},
abstract = {Hospital wastewater is a key interface between clinical and environmental reservoirs of antimicrobial resistance, fostering selection and horizontal gene transfer. Aeromonas spp. are aquatic opportunistic pathogens with highly plastic genomes and are increasingly recognized as potential intermediaries in resistance dissemination. We compared 72 cefiderocol-selected Aeromonas isolates recovered from untreated hospital wastewater collected at six tertiary care hospitals across Germany with 62 clinical isolates from patients with intestinal and extraintestinal infections, to characterize cefiderocol susceptibility, resistome composition, and genomic mobility features. Pangenome analysis revealed an open genome structure comprising 21,364 gene clusters, with a core genome of 2486 genes and a large cloud gene pool (15,612 clusters present in <15% of isolates), highlighting extensive genomic plasticity. Resistance phenotypes diverged markedly: cefiderocol-selected wastewater isolates exhibited high resistance rates to multiple clinically relevant agents - ciprofloxacin (93.1%), aztreonam (81.2%), and trimethoprim-sulfamethoxazole (38.9%), whereas clinical isolates remained largely susceptible overall (<10%). Under iron limitation, siderophore production increased in both cohorts; however, in the presence of cefiderocol it remained robust in wastewater isolates while being suppressed in clinical isolates. Comparative genomics showed that wastewater isolates carried substantially expanded resistomes (mean 13.8 ARGs; range 2-27) relative to clinical isolates (mean 2.6; range 1-11), including enrichment of clinically relevant β-lactamases and carbapenemases. This resistance burden coincided with a larger and more transmissible plasmidome and a high insertion sequence load. Notably, extensive plasmid-backbone homology was detected between Aeromonas and co-occurring cefiderocol-resistant Enterobacterales isolated from the same wastewater samples, highlighting interspecies gene flow at the hospital-environment interface. Together, these findings identify hospital wastewater as a reservoir and convergence point for highly resistant, mobilome-enriched Aeromonas subpopulations captured under cefiderocol selection, supporting Aeromonas as a One Health sentinel and emphasizing the value of wastewater-based surveillance for tracking mobile resistance determinants bridging environmental and clinical compartments.},
}
RevDate: 2026-02-05
CmpDate: 2026-02-05
Epidemiological and Genomic Insights into Linezolid-Non-Susceptible Enterococci in Pediatric Patients.
Current microbiology, 83(3):155.
Enterococci are major opportunistic pathogens causing healthcare-associated infections in children. Linezolid, a WHO-designated critically important antibiotic for multidrug-resistant Gram-positive infections, is increasingly challenged by linezolid-non-susceptible enterococci (LNSE). Yet pediatric LNSE epidemiology and genomics data remain scarce, hindering targeted control. We analyzed 26 LNSE strains isolated from Children's Hospital, Zhejiang University School of Medicine (June 2020-July 2024) using MALDI-TOF MS, Vitek2 Compact, micro-broth dilution (for linezolid MIC), MLST, resistance/virulence gene detection, and pan-genome analysis (COG/KEGG annotation). Enterococcus faecalis (E. faecalis) dominated (23/26,88.5%) with ST16 as the major sequence type (ST) and four novel STs identified; all strains harbored optrA and fexA, with species-specific resistance/virulence gene profiles. The 23 E. faecalis strains exhibited an open pan-genome (b = 0.174725), indicating the possible existence of active horizontal gene transfer (HGT), with core, accessory, and unique genes showing distinct functional differentiation. These findings provide critical and robust empirical data to inform the development of targeted prevention and control strategies against LNSE in pediatric populations.
Additional Links: PMID-41642358
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41642358,
year = {2026},
author = {Fang, C and Zhou, Z and Zhang, X and Xia, J and Liu, S and Li, J and Zhou, M},
title = {Epidemiological and Genomic Insights into Linezolid-Non-Susceptible Enterococci in Pediatric Patients.},
journal = {Current microbiology},
volume = {83},
number = {3},
pages = {155},
pmid = {41642358},
issn = {1432-0991},
support = {No. LTGC23H200006//Zhejiang Provincial Natural Science Foundation of China/ ; No. I23J0006//Key Program of The Independent Design Project of National Clinical Research Center for Child Health/ ; 2023C03028//"Pioneer" and "Leading Goose" R&D Program of Zhejiang Province/ ; },
mesh = {*Linezolid/pharmacology ; Humans ; *Gram-Positive Bacterial Infections/epidemiology/microbiology ; *Anti-Bacterial Agents/pharmacology ; Child ; Microbial Sensitivity Tests ; Genome, Bacterial ; *Enterococcus faecalis/genetics/drug effects/isolation & purification ; Multilocus Sequence Typing ; *Enterococcus/genetics/drug effects/isolation & purification/classification ; Genomics ; Child, Preschool ; China/epidemiology ; Drug Resistance, Multiple, Bacterial/genetics ; Infant ; },
abstract = {Enterococci are major opportunistic pathogens causing healthcare-associated infections in children. Linezolid, a WHO-designated critically important antibiotic for multidrug-resistant Gram-positive infections, is increasingly challenged by linezolid-non-susceptible enterococci (LNSE). Yet pediatric LNSE epidemiology and genomics data remain scarce, hindering targeted control. We analyzed 26 LNSE strains isolated from Children's Hospital, Zhejiang University School of Medicine (June 2020-July 2024) using MALDI-TOF MS, Vitek2 Compact, micro-broth dilution (for linezolid MIC), MLST, resistance/virulence gene detection, and pan-genome analysis (COG/KEGG annotation). Enterococcus faecalis (E. faecalis) dominated (23/26,88.5%) with ST16 as the major sequence type (ST) and four novel STs identified; all strains harbored optrA and fexA, with species-specific resistance/virulence gene profiles. The 23 E. faecalis strains exhibited an open pan-genome (b = 0.174725), indicating the possible existence of active horizontal gene transfer (HGT), with core, accessory, and unique genes showing distinct functional differentiation. These findings provide critical and robust empirical data to inform the development of targeted prevention and control strategies against LNSE in pediatric populations.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Linezolid/pharmacology
Humans
*Gram-Positive Bacterial Infections/epidemiology/microbiology
*Anti-Bacterial Agents/pharmacology
Child
Microbial Sensitivity Tests
Genome, Bacterial
*Enterococcus faecalis/genetics/drug effects/isolation & purification
Multilocus Sequence Typing
*Enterococcus/genetics/drug effects/isolation & purification/classification
Genomics
Child, Preschool
China/epidemiology
Drug Resistance, Multiple, Bacterial/genetics
Infant
RevDate: 2026-02-05
CmpDate: 2026-02-05
Molecular characterization of mcr-1.1-harboring multidrug-resistant Escherichia coli isolates from chicken in the United Arab Emirates: implications for one health surveillance.
Frontiers in veterinary science, 12:1714397.
BACKGROUND: The mcr-1.1 gene, conferring resistance to colistin, is a significant threat to public health, particularly due to its capacity for horizontal gene transfer between diverse bacterial populations in humans, animals, and the food chain. This study investigated the occurrence, phenotypic antimicrobial resistance (AMR) profiles, genetic characteristics, and plasmid characterization of mcr-1.1-producing Escherichia coli isolates from different samples in the United Arab Emirates (UAE).
METHODS: A total of 333 Gram-negative isolates were screened by PCR for the detection of mcr genes. Antimicrobial susceptibility testing, whole genome sequencing (WGS), plasmid analysis, and Phylogenomic typing were performed to assess AMR determinants, plasmid replicons, genetic contexts of mcr-1.1, and genetic relatedness between isolates from the UAE and neighboring countries.
RESULTS: We identified 15 mcr-1.1-positive E. coli strains, all from chicken cecal samples. These isolates exhibited multidrug resistance (MDR) to various classes of antibiotics, including β-lactams, tetracyclines, quinolones, and aminoglycosides. WGS of 15 mcr-positive E. coli isolates revealed the presence of multiple AMR genes along with mutations in quinolone resistance genes (gyrA, parC). Plasmid analysis revealed that all mcr-1.1-positive strains carried at least one plasmid replicon, with the IncF and IncI plasmids being the most prevalent. Notably, the mcr-1.1 gene was located on IncI2 and IncX4 plasmids, with comparative analysis showing high sequence homology to plasmids from E. coli strains originating from humans and animals in multiple countries. The plasmids' high sequence homology across diverse geographical regions provides genomic evidence consistent with possible cross-border dissemination of mcr-1.1, facilitating the spread of colistin resistance. Genetic mapping of the mcr-1.1 gene revealed distinct genetic contexts depending on the plasmid type, with genes such as nikA, nikB, and pap2 flanking the gene on IncI2 and IncX4 plasmids. Clonal analysis using whole-genome sequencing identified 12 different sequence types (STs) among the 15 isolates, with ST10, ST117, and ST162 being the most prevalent. Core genome multilocus sequence typing demonstrated genetic relatedness between isolates from the United Arab Emirates (UAE) and neighboring countries, indicating potential transmission across borders via the food chain.
CONCLUSION: Our findings highlight the complex interaction between plasmid-mediated colistin resistance, AMR, and virulence traits in E. coli from the food chain. The genetic and plasmid similarities between mcr-1.1-producing isolates across multiple countries emphasize the risk of possible dissemination and the potential risk of cross-border dissemination through globally traded food products. This study underscores the need for regional and global surveillance and control measures to mitigate the spread of this multidrug-resistant pathogen.
Additional Links: PMID-41640947
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41640947,
year = {2025},
author = {Khalifa, HO and Elbediwi, M and Mohammed, T and Abdalla, A and Mohamed, MI and Lakshmi, GB and Habib, I},
title = {Molecular characterization of mcr-1.1-harboring multidrug-resistant Escherichia coli isolates from chicken in the United Arab Emirates: implications for one health surveillance.},
journal = {Frontiers in veterinary science},
volume = {12},
number = {},
pages = {1714397},
pmid = {41640947},
issn = {2297-1769},
abstract = {BACKGROUND: The mcr-1.1 gene, conferring resistance to colistin, is a significant threat to public health, particularly due to its capacity for horizontal gene transfer between diverse bacterial populations in humans, animals, and the food chain. This study investigated the occurrence, phenotypic antimicrobial resistance (AMR) profiles, genetic characteristics, and plasmid characterization of mcr-1.1-producing Escherichia coli isolates from different samples in the United Arab Emirates (UAE).
METHODS: A total of 333 Gram-negative isolates were screened by PCR for the detection of mcr genes. Antimicrobial susceptibility testing, whole genome sequencing (WGS), plasmid analysis, and Phylogenomic typing were performed to assess AMR determinants, plasmid replicons, genetic contexts of mcr-1.1, and genetic relatedness between isolates from the UAE and neighboring countries.
RESULTS: We identified 15 mcr-1.1-positive E. coli strains, all from chicken cecal samples. These isolates exhibited multidrug resistance (MDR) to various classes of antibiotics, including β-lactams, tetracyclines, quinolones, and aminoglycosides. WGS of 15 mcr-positive E. coli isolates revealed the presence of multiple AMR genes along with mutations in quinolone resistance genes (gyrA, parC). Plasmid analysis revealed that all mcr-1.1-positive strains carried at least one plasmid replicon, with the IncF and IncI plasmids being the most prevalent. Notably, the mcr-1.1 gene was located on IncI2 and IncX4 plasmids, with comparative analysis showing high sequence homology to plasmids from E. coli strains originating from humans and animals in multiple countries. The plasmids' high sequence homology across diverse geographical regions provides genomic evidence consistent with possible cross-border dissemination of mcr-1.1, facilitating the spread of colistin resistance. Genetic mapping of the mcr-1.1 gene revealed distinct genetic contexts depending on the plasmid type, with genes such as nikA, nikB, and pap2 flanking the gene on IncI2 and IncX4 plasmids. Clonal analysis using whole-genome sequencing identified 12 different sequence types (STs) among the 15 isolates, with ST10, ST117, and ST162 being the most prevalent. Core genome multilocus sequence typing demonstrated genetic relatedness between isolates from the United Arab Emirates (UAE) and neighboring countries, indicating potential transmission across borders via the food chain.
CONCLUSION: Our findings highlight the complex interaction between plasmid-mediated colistin resistance, AMR, and virulence traits in E. coli from the food chain. The genetic and plasmid similarities between mcr-1.1-producing isolates across multiple countries emphasize the risk of possible dissemination and the potential risk of cross-border dissemination through globally traded food products. This study underscores the need for regional and global surveillance and control measures to mitigate the spread of this multidrug-resistant pathogen.},
}
RevDate: 2026-02-05
CmpDate: 2026-02-05
Editorial: Antimicrobial resistance: tracking and tackling in the food chain.
Frontiers in microbiology, 17:1769277.
Additional Links: PMID-41640431
Full Text:
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41640431,
year = {2026},
author = {Semedo-Lemsaddek, T and Jeon, B and González-Escalona, N and Laranjo, M},
title = {Editorial: Antimicrobial resistance: tracking and tackling in the food chain.},
journal = {Frontiers in microbiology},
volume = {17},
number = {},
pages = {1769277},
doi = {10.3389/fmicb.2026.1769277},
pmid = {41640431},
issn = {1664-302X},
}
RevDate: 2026-02-05
CmpDate: 2026-02-05
Comparative genomics reveals the genomic basis of race T2 emergence and heavy metal resistance in Xanthomonas euvesicatoria pv. perforans.
Frontiers in microbiology, 16:1718089.
Bacterial spot poses a significant threat to global pepper and tomato production. Recent phylogenomic analysis of whole genome sequences has revealed that solanaceous bacterial spot-causing xanthomonads belong to five distinct phylogenetic lineages within three species, including two pathovars within Xanthomonas euvesicatoria, X. hortorum pv. gardneri, and X. vesicatoria. X. euvesicatoria pv. perforans (Xep) strains are highly diverse and have become predominant in many tomato production regions. In this study, recently emerged Xep strains from Taiwan were assigned to tomato race T2 based on differential cultivar phenotyping, with effector genotyping used as supporting predictors. To clarify the genomic features of these Xep T2 strains, high-quality genome sequences of two representative isolates were generated and performed comparative genomic analyses were conducted. The T2 phenotype of these strains were supported by the absence and presence patterns of race-associated effector genes in the genome assemblies. Comparative analysis against published Xep genomes revealed plasmid diversity, the evolution of copper resistance, and signatures of horizontal gene transfer in these Xep T2 strains. Notably, a region containing a complete set of copper and heavy metal resistance genes was integrated into the chromosome, providing evidence on evolution of copper resistance in Xep strains in Taiwan. Accordingly, these findings suggest that horizontal gene transfer, including lysogenic conversion, and genetic recombination contribute to the ongoing diversification of X. euvesicatoria pv. perforans and may facilitate adaptation and persistence in tomato production agroecosystems.
Additional Links: PMID-41640400
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41640400,
year = {2025},
author = {Huang, CJ and Wu, TL and Lin, YH and Lin, YC},
title = {Comparative genomics reveals the genomic basis of race T2 emergence and heavy metal resistance in Xanthomonas euvesicatoria pv. perforans.},
journal = {Frontiers in microbiology},
volume = {16},
number = {},
pages = {1718089},
pmid = {41640400},
issn = {1664-302X},
abstract = {Bacterial spot poses a significant threat to global pepper and tomato production. Recent phylogenomic analysis of whole genome sequences has revealed that solanaceous bacterial spot-causing xanthomonads belong to five distinct phylogenetic lineages within three species, including two pathovars within Xanthomonas euvesicatoria, X. hortorum pv. gardneri, and X. vesicatoria. X. euvesicatoria pv. perforans (Xep) strains are highly diverse and have become predominant in many tomato production regions. In this study, recently emerged Xep strains from Taiwan were assigned to tomato race T2 based on differential cultivar phenotyping, with effector genotyping used as supporting predictors. To clarify the genomic features of these Xep T2 strains, high-quality genome sequences of two representative isolates were generated and performed comparative genomic analyses were conducted. The T2 phenotype of these strains were supported by the absence and presence patterns of race-associated effector genes in the genome assemblies. Comparative analysis against published Xep genomes revealed plasmid diversity, the evolution of copper resistance, and signatures of horizontal gene transfer in these Xep T2 strains. Notably, a region containing a complete set of copper and heavy metal resistance genes was integrated into the chromosome, providing evidence on evolution of copper resistance in Xep strains in Taiwan. Accordingly, these findings suggest that horizontal gene transfer, including lysogenic conversion, and genetic recombination contribute to the ongoing diversification of X. euvesicatoria pv. perforans and may facilitate adaptation and persistence in tomato production agroecosystems.},
}
RevDate: 2026-02-04
Accurate plasmid reconstruction from metagenomics data using assembly-alignment graphs and contrastive learning.
Nature biotechnology [Epub ahead of print].
Plasmids are extrachromosomal DNA molecules that enable horizontal gene transfer in bacteria, often conferring advantages such as antibiotic resistance. Despite their importance, plasmids are underrepresented in genomic databases because of challenges in assembling them, caused by mosaicism and microdiversity. Current plasmid assemblers rely on detecting circular paths in single-sample assembly graphs but face limitations because of graph fragmentation, entanglement and low coverage. We introduce PlasMAAG (plasmid and organism metagenomic binning using assembly-alignment graphs), a method to recover plasmids and cellular genomes from metagenomic samples. PlasMAAG complements assembly graph signals across samples by generating an 'assembly-alignment graph', which is used alongside common binning features for improved plasmid reconstruction. On synthetic benchmark datasets, PlasMAAG reconstructed 50-121% more near-complete plasmids than competing methods and improved the Matthews correlation coefficient of geNomad contig classification by 28-106%. On hospital sewage samples, PlasMAAG outperformed competing methods, reconstructing 33% more plasmid sequences. PlasMAAG enables the study of organism-plasmid associations and intraplasmid diversity across samples.
Additional Links: PMID-41639269
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41639269,
year = {2026},
author = {Piera Líndez, P and Danielsen, LS and Kovačić, I and Pielies Avellí, M and Nesme, J and Jensen, LJ and Andersen, JN and Sørensen, SJ and Rasmussen, S},
title = {Accurate plasmid reconstruction from metagenomics data using assembly-alignment graphs and contrastive learning.},
journal = {Nature biotechnology},
volume = {},
number = {},
pages = {},
pmid = {41639269},
issn = {1546-1696},
support = {NNF20OC0062223//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF14CC0001//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF23SA0084103//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF20OC0062223//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF23SA0084103//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF20OC0062223//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF14CC0001//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF20OC0062223//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF20OC0062223//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF23SA0084103//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; NNF20OC0062223//Novo Nordisk Fonden (Novo Nordisk Foundation)/ ; },
abstract = {Plasmids are extrachromosomal DNA molecules that enable horizontal gene transfer in bacteria, often conferring advantages such as antibiotic resistance. Despite their importance, plasmids are underrepresented in genomic databases because of challenges in assembling them, caused by mosaicism and microdiversity. Current plasmid assemblers rely on detecting circular paths in single-sample assembly graphs but face limitations because of graph fragmentation, entanglement and low coverage. We introduce PlasMAAG (plasmid and organism metagenomic binning using assembly-alignment graphs), a method to recover plasmids and cellular genomes from metagenomic samples. PlasMAAG complements assembly graph signals across samples by generating an 'assembly-alignment graph', which is used alongside common binning features for improved plasmid reconstruction. On synthetic benchmark datasets, PlasMAAG reconstructed 50-121% more near-complete plasmids than competing methods and improved the Matthews correlation coefficient of geNomad contig classification by 28-106%. On hospital sewage samples, PlasMAAG outperformed competing methods, reconstructing 33% more plasmid sequences. PlasMAAG enables the study of organism-plasmid associations and intraplasmid diversity across samples.},
}
RevDate: 2026-02-04
Nationwide emergence of cefotaxime-resistant Neisseria meningitidis via interspecies gene transfer from penA795-bearing Neisseria commensals in China.
Additional Links: PMID-41638474
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41638474,
year = {2026},
author = {Shao, Y and Lan, X and Chen, M and Wang, M and Guo, Q},
title = {Nationwide emergence of cefotaxime-resistant Neisseria meningitidis via interspecies gene transfer from penA795-bearing Neisseria commensals in China.},
journal = {Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases},
volume = {},
number = {},
pages = {},
doi = {10.1016/j.cmi.2026.01.026},
pmid = {41638474},
issn = {1469-0691},
}
RevDate: 2026-02-04
CmpDate: 2026-02-04
Plant-fungi interactions in Marchantia polymorpha are associated with horizontal gene transfer and terpene metabolism.
Proceedings of the National Academy of Sciences of the United States of America, 123(6):e2532723123.
The liverwort Marchantia polymorpha has emerged as a model for studying plant immunity in bryophytes, providing unique insights into conserved defense mechanisms across land plants. By contrast, Marchantia-specific immune mechanisms remained largely underexplored. In this study, we investigated the genetic basis of quantitative resistance in M. polymorpha against the fungal pathogen Colletotrichum nymphaeae, a naturally occurring compatible parasite. Through a combination of phenotypic, cytological, and transcriptomic approaches, combined with genome-wide association studies (GWAS), we identified key defense-related genes and pathways. Leveraging the biological and genetic variability present in a collection of natural M. polymorpha accessions, we highlight the role of horizontally transferred microbial-like terpene synthase genes, which may contribute to the exceptional terpene diversity of liverworts and potentially play a role in pathogen resistance. GWAS uncovered candidate loci associated with resistance traits, implicating both core immune components and specialized metabolic pathways. Transcriptomic analyses performed on two accessions with contrasting phenotypes after inoculation with C. nymphaeae revealed the upregulation of accession-specific and horizontally acquired genes. These results provide insights into the specific molecular underpinnings of bryophyte immunity and underscore the evolutionary significance of horizontal gene transfer and specialized metabolites in shaping plant-pathogen interactions.
Additional Links: PMID-41637459
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41637459,
year = {2026},
author = {El Mahboubi, K and Beaulieu, C and Castel, B and Libourel, C and Jariais, N and Amblard, E and van Beveren, F and Keller, J and Martinez, Y and Nelson, JM and Bonhomme, M and Jacquet, C and Delaux, PM},
title = {Plant-fungi interactions in Marchantia polymorpha are associated with horizontal gene transfer and terpene metabolism.},
journal = {Proceedings of the National Academy of Sciences of the United States of America},
volume = {123},
number = {6},
pages = {e2532723123},
doi = {10.1073/pnas.2532723123},
pmid = {41637459},
issn = {1091-6490},
support = {ANR-10-LABX-41//Agence Nationale de la Recherche (ANR)/ ; 101001675 - ORIGINS//EC | European Research Council (ERC)/ ; FRM/FSER202302017064//Fondation pour la Recherche Médicale (FRM)/ ; 80|PRIME MicMac//Centre National de la Recherche Scientifique (CNRS)/ ; ANR-21-CE20-0010-01//Agence Nationale de la Recherche (ANR)/ ; },
mesh = {*Gene Transfer, Horizontal ; *Marchantia/microbiology/genetics/metabolism/immunology ; Genome-Wide Association Study ; *Terpenes/metabolism ; *Colletotrichum/pathogenicity/physiology ; *Plant Diseases/microbiology/genetics ; *Host-Pathogen Interactions/genetics ; Disease Resistance/genetics ; Plant Immunity/genetics ; Gene Expression Regulation, Plant ; Plant Proteins/genetics/metabolism ; Transcriptome ; },
abstract = {The liverwort Marchantia polymorpha has emerged as a model for studying plant immunity in bryophytes, providing unique insights into conserved defense mechanisms across land plants. By contrast, Marchantia-specific immune mechanisms remained largely underexplored. In this study, we investigated the genetic basis of quantitative resistance in M. polymorpha against the fungal pathogen Colletotrichum nymphaeae, a naturally occurring compatible parasite. Through a combination of phenotypic, cytological, and transcriptomic approaches, combined with genome-wide association studies (GWAS), we identified key defense-related genes and pathways. Leveraging the biological and genetic variability present in a collection of natural M. polymorpha accessions, we highlight the role of horizontally transferred microbial-like terpene synthase genes, which may contribute to the exceptional terpene diversity of liverworts and potentially play a role in pathogen resistance. GWAS uncovered candidate loci associated with resistance traits, implicating both core immune components and specialized metabolic pathways. Transcriptomic analyses performed on two accessions with contrasting phenotypes after inoculation with C. nymphaeae revealed the upregulation of accession-specific and horizontally acquired genes. These results provide insights into the specific molecular underpinnings of bryophyte immunity and underscore the evolutionary significance of horizontal gene transfer and specialized metabolites in shaping plant-pathogen interactions.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Gene Transfer, Horizontal
*Marchantia/microbiology/genetics/metabolism/immunology
Genome-Wide Association Study
*Terpenes/metabolism
*Colletotrichum/pathogenicity/physiology
*Plant Diseases/microbiology/genetics
*Host-Pathogen Interactions/genetics
Disease Resistance/genetics
Plant Immunity/genetics
Gene Expression Regulation, Plant
Plant Proteins/genetics/metabolism
Transcriptome
RevDate: 2026-02-04
Genomic and functional dissection of natural transformation-related genes in Piscirickettsia salmonis.
Microbiology spectrum [Epub ahead of print].
Piscirickettsia salmonis, the etiological agent of piscirickettsiosis, represents the main health challenge for Chilean salmon farming and an emerging threat to global salmonid production. Inspection of all P. salmonis genomes available shows that the comEC gene, encoding the DNA uptake channel required for natural transformation (NT) in competent bacteria, is interrupted by various transposable elements, whereas other NT determinants (comEA, comFB, comM, comL/bamD, and dprA) remain intact and highly conserved. Using P. salmonis Psal-103 (EM-genogroup) and Psal-104b (LF-genogroup) as representatives of the two most prevalent genogroups in Chile, we combined comparative genomics, gene expression analysis, heterologous expression of comEC using homologs from naturally competent bacteria, and CRISPRi-mediated knockdown of the remaining NT-related genes to examine their functionality. According to our results, expression of comEC with homologs from Legionella pneumophila and Vibrio cholerae impaired P. salmonis viability in axenic culture, indicating a fitness burden. CRISPRi-mediated repression of comL/bamD revealed its essentiality in Psal-104b and a critical role during infection of the SHK-1 cell line in Psal-103. Repression of comM tended to reduce cytopathogenicity in both strains, while repression of comFB was associated with a modest delay in cytopathic damage in Psal-104b. Repression of recA and comEA caused moderate reductions in cytopathic activity in Psal-103. By contrast, dprA showed no detectable phenotypes. Together, our results indicate that despite the irreversible interruption of comEC, P. salmonis has retained NT-related genes that contribute to fitness in a gene- and genogroup-specific manner, providing a framework to investigate how determinants of horizontal gene transfer function beyond DNA uptake.IMPORTANCEDespite its major impact on salmon aquaculture, Piscirickettsia salmonis remains poorly characterized at the functional level, largely due to long-standing limitations in genetic tractability. Here, we implement and combine multiple genetic approaches, including CRISPR interference, site-specific chromosomal integration, and heterologous gene expression, to functionally interrogate natural transformation (NT)-related genes in this pathogen. Using representative strains from the two most prevalent Chilean genogroups, we show that conserved NT-associated genes contribute to bacterial physiology and infection in a genogroup-dependent manner. Moreover, and beyond the specific biological findings, this work establishes a versatile genetic platform for functional studies in P. salmonis, expanding the experimental toolbox available to study this pathogen and supporting future efforts aimed at understanding its biology and the development of novel biotechnological approaches.
Additional Links: PMID-41636501
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41636501,
year = {2026},
author = {Higuera-Llantén, S and Ojeda, N and Protz, J and Marshall, SH},
title = {Genomic and functional dissection of natural transformation-related genes in Piscirickettsia salmonis.},
journal = {Microbiology spectrum},
volume = {},
number = {},
pages = {e0317325},
doi = {10.1128/spectrum.03173-25},
pmid = {41636501},
issn = {2165-0497},
abstract = {Piscirickettsia salmonis, the etiological agent of piscirickettsiosis, represents the main health challenge for Chilean salmon farming and an emerging threat to global salmonid production. Inspection of all P. salmonis genomes available shows that the comEC gene, encoding the DNA uptake channel required for natural transformation (NT) in competent bacteria, is interrupted by various transposable elements, whereas other NT determinants (comEA, comFB, comM, comL/bamD, and dprA) remain intact and highly conserved. Using P. salmonis Psal-103 (EM-genogroup) and Psal-104b (LF-genogroup) as representatives of the two most prevalent genogroups in Chile, we combined comparative genomics, gene expression analysis, heterologous expression of comEC using homologs from naturally competent bacteria, and CRISPRi-mediated knockdown of the remaining NT-related genes to examine their functionality. According to our results, expression of comEC with homologs from Legionella pneumophila and Vibrio cholerae impaired P. salmonis viability in axenic culture, indicating a fitness burden. CRISPRi-mediated repression of comL/bamD revealed its essentiality in Psal-104b and a critical role during infection of the SHK-1 cell line in Psal-103. Repression of comM tended to reduce cytopathogenicity in both strains, while repression of comFB was associated with a modest delay in cytopathic damage in Psal-104b. Repression of recA and comEA caused moderate reductions in cytopathic activity in Psal-103. By contrast, dprA showed no detectable phenotypes. Together, our results indicate that despite the irreversible interruption of comEC, P. salmonis has retained NT-related genes that contribute to fitness in a gene- and genogroup-specific manner, providing a framework to investigate how determinants of horizontal gene transfer function beyond DNA uptake.IMPORTANCEDespite its major impact on salmon aquaculture, Piscirickettsia salmonis remains poorly characterized at the functional level, largely due to long-standing limitations in genetic tractability. Here, we implement and combine multiple genetic approaches, including CRISPR interference, site-specific chromosomal integration, and heterologous gene expression, to functionally interrogate natural transformation (NT)-related genes in this pathogen. Using representative strains from the two most prevalent Chilean genogroups, we show that conserved NT-associated genes contribute to bacterial physiology and infection in a genogroup-dependent manner. Moreover, and beyond the specific biological findings, this work establishes a versatile genetic platform for functional studies in P. salmonis, expanding the experimental toolbox available to study this pathogen and supporting future efforts aimed at understanding its biology and the development of novel biotechnological approaches.},
}
RevDate: 2026-02-04
CmpDate: 2026-02-04
Targeting anti-virulence factor strategies of bacterial pathogens.
Biosafety and health, 7(1):1-4.
Antibiotic-resistant bacterial pathogens pose substantial biosafety and health hazards, leading to millions of deaths each year. The evolution of bacterial virulence factors is mainly propelled by horizontal gene transfer (HGT). In addition to traditional antibiotics, antimicrobial strategies targeting biofilm-related virulence factors and quorum sensing (QS)-related virulence factors can effectively restrain drug-resistant bacteria. Future anti-virulence strategies, encompassing natural drugs, antibiotic resistance inhibitors, monoclonal antibodies (mAbs), and vaccines, are in the development pipeline. Consequently, by disrupting virulence factors, these drugs can eliminate the ability of bacterial pathogens to cause disease. In conclusion, this Perspective comprehensively summarizes current anti-bacterial virulence factor strategies and prospects for future cutting-edge approaches, which may address the issues of antibacterial resistance and curtail the spread of pathogens in the future.
Additional Links: PMID-41635302
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41635302,
year = {2025},
author = {Li, J and Jia, T and Yang, L},
title = {Targeting anti-virulence factor strategies of bacterial pathogens.},
journal = {Biosafety and health},
volume = {7},
number = {1},
pages = {1-4},
pmid = {41635302},
issn = {2590-0536},
abstract = {Antibiotic-resistant bacterial pathogens pose substantial biosafety and health hazards, leading to millions of deaths each year. The evolution of bacterial virulence factors is mainly propelled by horizontal gene transfer (HGT). In addition to traditional antibiotics, antimicrobial strategies targeting biofilm-related virulence factors and quorum sensing (QS)-related virulence factors can effectively restrain drug-resistant bacteria. Future anti-virulence strategies, encompassing natural drugs, antibiotic resistance inhibitors, monoclonal antibodies (mAbs), and vaccines, are in the development pipeline. Consequently, by disrupting virulence factors, these drugs can eliminate the ability of bacterial pathogens to cause disease. In conclusion, this Perspective comprehensively summarizes current anti-bacterial virulence factor strategies and prospects for future cutting-edge approaches, which may address the issues of antibacterial resistance and curtail the spread of pathogens in the future.},
}
RevDate: 2026-02-03
Assembly and comparative analysis of the complete mitochondrial genome of two species of Argentina (Rosaceae).
BMC genomics pii:10.1186/s12864-025-12442-8 [Epub ahead of print].
BACKGROUND: Argentina anserina and Argentina lineata are alpine plant species endemic to the Qinghai-Tibet Plateau (QTP). However, the dynamic features of their mitochondrial genome characteristics remain poorly characterized.
METHODS: We conducted de novo assembly and annotation of the mitochondrial genomes of two Argentina species using PacBio HiFi and Illumina sequencing technologies.
RESULTS: The mitochondrial genomes of A. anserina and A. lineata both exhibit a single circular structure, with sizes of 294,533 bp and 338,624 bp, respectively. Both genomes encode 30 protein-coding genes (PCGs) and 3 ribosomal RNA (rRNA) genes, but differ in the number of transfer RNA (tRNA) genes (18 vs. 19), with A. lineata harboring the unique trnS-UGA. Codons exhibit a preference for A/U endings, consistent with their respective genomic GC contents (44.48% and 43.98%). A total of 217 high-confidence RNA editing sites were detected in A. anserina and 209 in A. lineata, with the majority of these edits leading to hydrophobic amino acid substitutions. Experimental validation confirmed RNA editing at four target sites (i.e., nad1-2, nad4L-2, atp6-718, and ccmFC-1312) in A. anserina. Horizontal gene transfer (HGT) analysis identified 20 and 29 chloroplast derived sequences in mitochondrial genomes of A. anserina and A. lineata, respectively, including the complete trnD-GUC gene and fragments of atpB, rpoC1, and rpoC2 genes, which contributes to the remodeling of energy metabolic pathways. Phylogenetic analysis indicated that the genus Argentina is more closely related to Potentilla than to Fragaria, and synteny analysis further revealed genomic structural divergence among these genera.
CONCLUSIONS: This study elucidates the potential roles of RNA editing and HGT events in the mitochondrial genome evolution of the two Argentina species, and furnishes valuable mitochondrial genomic resources for alpine plant research.
Additional Links: PMID-41634547
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41634547,
year = {2026},
author = {Tian, Z and Wu, X and Zhang, T and Qiong, L},
title = {Assembly and comparative analysis of the complete mitochondrial genome of two species of Argentina (Rosaceae).},
journal = {BMC genomics},
volume = {},
number = {},
pages = {},
doi = {10.1186/s12864-025-12442-8},
pmid = {41634547},
issn = {1471-2164},
support = {2021-GSP-B019//High-level graduate research project, Xizang University/ ; 31760127//National Natural Science Foundation of China/ ; 00060906-01//a first-class discipline construction project in ecology/ ; XZ202402ZY0023//the Science and Technology Program of Xizang Autonomous Region/ ; },
abstract = {BACKGROUND: Argentina anserina and Argentina lineata are alpine plant species endemic to the Qinghai-Tibet Plateau (QTP). However, the dynamic features of their mitochondrial genome characteristics remain poorly characterized.
METHODS: We conducted de novo assembly and annotation of the mitochondrial genomes of two Argentina species using PacBio HiFi and Illumina sequencing technologies.
RESULTS: The mitochondrial genomes of A. anserina and A. lineata both exhibit a single circular structure, with sizes of 294,533 bp and 338,624 bp, respectively. Both genomes encode 30 protein-coding genes (PCGs) and 3 ribosomal RNA (rRNA) genes, but differ in the number of transfer RNA (tRNA) genes (18 vs. 19), with A. lineata harboring the unique trnS-UGA. Codons exhibit a preference for A/U endings, consistent with their respective genomic GC contents (44.48% and 43.98%). A total of 217 high-confidence RNA editing sites were detected in A. anserina and 209 in A. lineata, with the majority of these edits leading to hydrophobic amino acid substitutions. Experimental validation confirmed RNA editing at four target sites (i.e., nad1-2, nad4L-2, atp6-718, and ccmFC-1312) in A. anserina. Horizontal gene transfer (HGT) analysis identified 20 and 29 chloroplast derived sequences in mitochondrial genomes of A. anserina and A. lineata, respectively, including the complete trnD-GUC gene and fragments of atpB, rpoC1, and rpoC2 genes, which contributes to the remodeling of energy metabolic pathways. Phylogenetic analysis indicated that the genus Argentina is more closely related to Potentilla than to Fragaria, and synteny analysis further revealed genomic structural divergence among these genera.
CONCLUSIONS: This study elucidates the potential roles of RNA editing and HGT events in the mitochondrial genome evolution of the two Argentina species, and furnishes valuable mitochondrial genomic resources for alpine plant research.},
}
RevDate: 2026-02-03
Unraveling the sources and influencing mechanism of soil antibiotic resistance genes in urban micro green spaces.
Environmental science. Processes & impacts [Epub ahead of print].
Characterized by the small size and extensive distribution, micro green spaces are vital for urban environmental quality and resident well-being. Yet, they are increasingly recognized as hotspots for the convergence of antibiotic resistance genes (ARGs); systematic research on ARG pollution in these areas remains limited. This study investigated the distribution and sources of ARGs in soils from 21 micro green spaces in Tianjin, China. The results indicated a high prevalence of ARGs, with a predominance of aminoglycoside, β-lactam, fluoroquinolone and multidrug resistance genes. Their dissemination was primarily facilitated by protection mechanisms and horizontal gene transfer (HGT) mediated by mobile genetic elements (MGEs). Source analysis indicated that in intra-urban areas, ARGs were mainly contributed by trash (46.9%), followed by irrigation water (37.3%) and pet/bird feces (15.8%). In extra-urban areas, irrigation water was the dominant source (72.8%), demonstrating considerable spatial heterogeneity. Mechanistic analysis revealed soil total phosphorus (TP) as the strongest driver of ARG enrichment (p < 0.001). Furthermore, specific phyla like Cloacimonadota and Myxococcota were linked to ARG diffusion through their correlation with MGEs. This study fills a key knowledge gap on ARGs in micro green spaces, providing a scientific basis for interventions aimed at safeguarding urban ecological security and public health.
Additional Links: PMID-41631630
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41631630,
year = {2026},
author = {Li, Q and Lv, L and Wu, J and He, J and Pang, M and He, M and Zhang, H},
title = {Unraveling the sources and influencing mechanism of soil antibiotic resistance genes in urban micro green spaces.},
journal = {Environmental science. Processes & impacts},
volume = {},
number = {},
pages = {},
doi = {10.1039/d5em00851d},
pmid = {41631630},
issn = {2050-7895},
abstract = {Characterized by the small size and extensive distribution, micro green spaces are vital for urban environmental quality and resident well-being. Yet, they are increasingly recognized as hotspots for the convergence of antibiotic resistance genes (ARGs); systematic research on ARG pollution in these areas remains limited. This study investigated the distribution and sources of ARGs in soils from 21 micro green spaces in Tianjin, China. The results indicated a high prevalence of ARGs, with a predominance of aminoglycoside, β-lactam, fluoroquinolone and multidrug resistance genes. Their dissemination was primarily facilitated by protection mechanisms and horizontal gene transfer (HGT) mediated by mobile genetic elements (MGEs). Source analysis indicated that in intra-urban areas, ARGs were mainly contributed by trash (46.9%), followed by irrigation water (37.3%) and pet/bird feces (15.8%). In extra-urban areas, irrigation water was the dominant source (72.8%), demonstrating considerable spatial heterogeneity. Mechanistic analysis revealed soil total phosphorus (TP) as the strongest driver of ARG enrichment (p < 0.001). Furthermore, specific phyla like Cloacimonadota and Myxococcota were linked to ARG diffusion through their correlation with MGEs. This study fills a key knowledge gap on ARGs in micro green spaces, providing a scientific basis for interventions aimed at safeguarding urban ecological security and public health.},
}
RevDate: 2026-02-02
CmpDate: 2026-02-02
Therapeutic milestones against multidrug resistant Acinetobacter baumannii: from legacy antibiotics to Zosurabalpin.
Archives of microbiology, 208(4):177.
Antimicrobial resistance (AMR) in Acinetobacter baumannii represents a critical global health challenge, particularly in intensive care settings where the pathogen causes severe, refractory infections. As a leading member of the ESKAPE group, A. baumannii has accumulated extensive resistance to multiple antibiotic classes, including carbapenems, resulting in the widespread emergence of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) strains. This review provides a chronological overview of the evolution of antimicrobial therapies used against A. baumannii, spanning the early era of penicillins and tetracyclines to contemporary agents such as eravacycline and ceftazidime-avibactam. We delineate the molecular mechanisms underlying resistance development, including carbapenemase production, robust RND efflux systems, horizontal gene transfer, biofilm formation, and the global dissemination of high-risk international clones (IC1-IC9). The compounding impact of the COVID-19 pandemic on the spread of carbapenem-resistant A. baumannii (CRAB) is also examined. A special emphasis is placed on Zosurabalpin, a first-in-class macrocyclic peptide antibiotic with a unique mechanism of action that targets the LptB2FG complex essential for lipooligosaccharide (LOS) transport and outer membrane assembly. Preclinical data and emerging clinical findings highlight its potent activity against highly resistant CRAB strains and its ability to circumvent conventional resistance pathways, marking it as a promising candidate in the antimicrobial pipeline. Finally, we evaluate the limitations of current treatment modalities and explore emerging strategies, including phage therapy, novel target discovery, and non-traditional therapeutics, offering a forward-looking perspective on restoring and sustaining effective anti-Acinetobacter interventions.
Additional Links: PMID-41627487
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41627487,
year = {2026},
author = {Malik, J and Singh, S and Shrivastav, D and Verma, VV and Pal, RK and Mishra, MK and Sharma, VK},
title = {Therapeutic milestones against multidrug resistant Acinetobacter baumannii: from legacy antibiotics to Zosurabalpin.},
journal = {Archives of microbiology},
volume = {208},
number = {4},
pages = {177},
pmid = {41627487},
issn = {1432-072X},
mesh = {*Acinetobacter baumannii/drug effects/genetics ; *Drug Resistance, Multiple, Bacterial/drug effects ; *Anti-Bacterial Agents/therapeutic use/pharmacology ; Humans ; *Acinetobacter Infections/drug therapy/microbiology ; },
abstract = {Antimicrobial resistance (AMR) in Acinetobacter baumannii represents a critical global health challenge, particularly in intensive care settings where the pathogen causes severe, refractory infections. As a leading member of the ESKAPE group, A. baumannii has accumulated extensive resistance to multiple antibiotic classes, including carbapenems, resulting in the widespread emergence of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) strains. This review provides a chronological overview of the evolution of antimicrobial therapies used against A. baumannii, spanning the early era of penicillins and tetracyclines to contemporary agents such as eravacycline and ceftazidime-avibactam. We delineate the molecular mechanisms underlying resistance development, including carbapenemase production, robust RND efflux systems, horizontal gene transfer, biofilm formation, and the global dissemination of high-risk international clones (IC1-IC9). The compounding impact of the COVID-19 pandemic on the spread of carbapenem-resistant A. baumannii (CRAB) is also examined. A special emphasis is placed on Zosurabalpin, a first-in-class macrocyclic peptide antibiotic with a unique mechanism of action that targets the LptB2FG complex essential for lipooligosaccharide (LOS) transport and outer membrane assembly. Preclinical data and emerging clinical findings highlight its potent activity against highly resistant CRAB strains and its ability to circumvent conventional resistance pathways, marking it as a promising candidate in the antimicrobial pipeline. Finally, we evaluate the limitations of current treatment modalities and explore emerging strategies, including phage therapy, novel target discovery, and non-traditional therapeutics, offering a forward-looking perspective on restoring and sustaining effective anti-Acinetobacter interventions.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Acinetobacter baumannii/drug effects/genetics
*Drug Resistance, Multiple, Bacterial/drug effects
*Anti-Bacterial Agents/therapeutic use/pharmacology
Humans
*Acinetobacter Infections/drug therapy/microbiology
RevDate: 2026-02-02
Genome contamination may lead to an overestimation of horizontal gene transfer inferences.
Nature communications, 17(1):1219.
Additional Links: PMID-41629337
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41629337,
year = {2026},
author = {Godron, N and Ruppé, E and Leclercq, SO},
title = {Genome contamination may lead to an overestimation of horizontal gene transfer inferences.},
journal = {Nature communications},
volume = {17},
number = {1},
pages = {1219},
pmid = {41629337},
issn = {2041-1723},
}
RevDate: 2026-02-02
CmpDate: 2026-02-02
Frequency of CIT, EBC and DHA flocks (families) of AmpC beta-lactamases in clinical isolates of Klebsiella pneumoniae collected from hospitalized patients in North of Iran.
Molecular biology reports, 53(1):346.
BACKGROUND: Klebsiella pneumoniae exhibits a marked propensity for acquiring beta-lactam resistance genes. Among these, AmpC beta-lactamases are able to hydrolyze a broad spectrum of antibiotics including first- through third-generation cephalosporins, cephamycins, and aztreonam. The CIT, EBC, and DHA families are among the most clinically significant plasmid-mediated AmpC variants in this pathogen. Consequently, this study aimed to investigate the prevalence of genes encoding these specific enzymes among clinical isolates of K. pneumoniae in northern Iran.
METHODS: One hundred clinical isolates were collected from hospitalized patients and identified using standard microbiological and biochemical assays. The antimicrobial resistance profile of each isolate was determined via the disk agar diffusion method. Subsequently, PCR test was employed to detect the presence of the blaCIT, blaEBC, and blaDHA genes.
RESULTS: The mean age of the patients (58 females and 42 males) was 50.31 years. Isolates were sourced from patients in general (41%), pediatric (45%), burn (7%), and infectious (7%) hospitals. The primary specimen sources were urine (64%), blood (10%), tissue (15%), wound (7%), and sputum (4%). The highest prevalence of resistance (93%) was observed against ampicillin-sulbactam, whereas 73% of isolates remained susceptible to ertapenem.
CONCLUSION: The high ampicillin-sulbactam resistance represents a serious concern for the management of hospital-acquired infections. Furthermore, while the presence of the investigated blaCIT, blaEBC, and blaDHA genes did not show a statistically significant correlation with resistance to most tested antibiotics, their detection remains of potential clinical importance due to the risk of horizontal gene transfer to other bacterial species.
Additional Links: PMID-41627608
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41627608,
year = {2026},
author = {Betiar, F and Gholami, M and Karimbakhsh, M and Samadi, M and Elahi, F and Goli, HR},
title = {Frequency of CIT, EBC and DHA flocks (families) of AmpC beta-lactamases in clinical isolates of Klebsiella pneumoniae collected from hospitalized patients in North of Iran.},
journal = {Molecular biology reports},
volume = {53},
number = {1},
pages = {346},
pmid = {41627608},
issn = {1573-4978},
mesh = {Humans ; *Klebsiella pneumoniae/genetics/isolation & purification/drug effects/enzymology ; *beta-Lactamases/genetics/metabolism ; Male ; Iran ; Female ; *Bacterial Proteins/genetics/metabolism ; Middle Aged ; *Klebsiella Infections/microbiology/drug therapy ; Adult ; Anti-Bacterial Agents/pharmacology ; Microbial Sensitivity Tests ; Aged ; Hospitalization ; beta-Lactam Resistance/genetics ; },
abstract = {BACKGROUND: Klebsiella pneumoniae exhibits a marked propensity for acquiring beta-lactam resistance genes. Among these, AmpC beta-lactamases are able to hydrolyze a broad spectrum of antibiotics including first- through third-generation cephalosporins, cephamycins, and aztreonam. The CIT, EBC, and DHA families are among the most clinically significant plasmid-mediated AmpC variants in this pathogen. Consequently, this study aimed to investigate the prevalence of genes encoding these specific enzymes among clinical isolates of K. pneumoniae in northern Iran.
METHODS: One hundred clinical isolates were collected from hospitalized patients and identified using standard microbiological and biochemical assays. The antimicrobial resistance profile of each isolate was determined via the disk agar diffusion method. Subsequently, PCR test was employed to detect the presence of the blaCIT, blaEBC, and blaDHA genes.
RESULTS: The mean age of the patients (58 females and 42 males) was 50.31 years. Isolates were sourced from patients in general (41%), pediatric (45%), burn (7%), and infectious (7%) hospitals. The primary specimen sources were urine (64%), blood (10%), tissue (15%), wound (7%), and sputum (4%). The highest prevalence of resistance (93%) was observed against ampicillin-sulbactam, whereas 73% of isolates remained susceptible to ertapenem.
CONCLUSION: The high ampicillin-sulbactam resistance represents a serious concern for the management of hospital-acquired infections. Furthermore, while the presence of the investigated blaCIT, blaEBC, and blaDHA genes did not show a statistically significant correlation with resistance to most tested antibiotics, their detection remains of potential clinical importance due to the risk of horizontal gene transfer to other bacterial species.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
Humans
*Klebsiella pneumoniae/genetics/isolation & purification/drug effects/enzymology
*beta-Lactamases/genetics/metabolism
Male
Iran
Female
*Bacterial Proteins/genetics/metabolism
Middle Aged
*Klebsiella Infections/microbiology/drug therapy
Adult
Anti-Bacterial Agents/pharmacology
Microbial Sensitivity Tests
Aged
Hospitalization
beta-Lactam Resistance/genetics
RevDate: 2026-02-02
Serratia species as paratransgenic vehicles: potential applications in vector-borne disease control.
Clinical microbiology reviews [Epub ahead of print].
SUMMARYParatransgenesis employs insect-associated bacteria to deliver antipathogen effectors and is an emergent complementary strategy for vector control. This review synthesizes current evidence for Serratia species as paratransgenic vehicles, combining mechanistic insights into effector molecules (e.g., scorpine, MP2, multi-fusion constructs, and the naturally secreted antimalarial lipase AmLip), with comparative evidence on colonization, transmission, and efficacy. Serratia strains (e.g., AS1, Su_YN1) demonstrate rapid dissemination in laboratory populations and potent reductions in Plasmodium development (reported oocyst inhibition in laboratory studies ranging from ~60% to >90% for specific effectors). We critically examine biosafety, genetic stability, and ecological factors and propose a minimum evidence package and translational roadmap comprising multigeneration stability assays, horizontal gene transfer monitoring, non-target impact assessments, and community and regulatory engagement to responsibly advance Serratia-based paratransgenesis toward field evaluation. This comparative framing integrates Serratia-focused detail with the broader paratransgenesis literature to clarify both its promise and remaining knowledge gaps.
Additional Links: PMID-41627026
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41627026,
year = {2026},
author = {Mahor, S and Gupta, H},
title = {Serratia species as paratransgenic vehicles: potential applications in vector-borne disease control.},
journal = {Clinical microbiology reviews},
volume = {},
number = {},
pages = {e0028025},
doi = {10.1128/cmr.00280-25},
pmid = {41627026},
issn = {1098-6618},
abstract = {SUMMARYParatransgenesis employs insect-associated bacteria to deliver antipathogen effectors and is an emergent complementary strategy for vector control. This review synthesizes current evidence for Serratia species as paratransgenic vehicles, combining mechanistic insights into effector molecules (e.g., scorpine, MP2, multi-fusion constructs, and the naturally secreted antimalarial lipase AmLip), with comparative evidence on colonization, transmission, and efficacy. Serratia strains (e.g., AS1, Su_YN1) demonstrate rapid dissemination in laboratory populations and potent reductions in Plasmodium development (reported oocyst inhibition in laboratory studies ranging from ~60% to >90% for specific effectors). We critically examine biosafety, genetic stability, and ecological factors and propose a minimum evidence package and translational roadmap comprising multigeneration stability assays, horizontal gene transfer monitoring, non-target impact assessments, and community and regulatory engagement to responsibly advance Serratia-based paratransgenesis toward field evaluation. This comparative framing integrates Serratia-focused detail with the broader paratransgenesis literature to clarify both its promise and remaining knowledge gaps.},
}
RevDate: 2026-02-02
CmpDate: 2026-02-02
Wilson's disease-associated gut dysbiosis: novel insights into microbial functional alterations, virulence changes, and resistance markers.
Frontiers in microbiology, 16:1714276.
BACKGROUND: Although the gut microbiota is associated with a variety of metabolic, inflammatory, and neurological disorders through microbial dysbiosis, current studies on the gut microbiota in Wilson's disease (WD) remain limited. Critical gaps exist in understanding the roles of key functional microbial factors in WD pathogenesis, which hinders the acquisition of mechanistic insights into this disease.
OBJECTIVE: This study aims to characterize alterations in the gut microbiome associated with WD, with a particular emphasis on virulence factors (VFs) and antibiotic resistance genes (ARGs), as well as functional mobile genetic elements (MGEs), in order to elucidate their potential roles in disease progression and clinical manifestations.
METHODS: We analyzed fecal samples from 37 patients with WD and 33 healthy controls (HCs) using metagenomic sequencing, with a specific focus on examining virulence gene profiles and antibiotic resistance patterns and MGE composition in relation to liver function markers.
RESULTS: Beta diversity analysis revealed significant differences in the gut microbial community structure between patients with WD and HCs, and a distinct set of microbial taxa was identified that showed significant associations with clinical indicators. A gut microbial co-occurrence network identified key species playing central roles in the microbial community structure, including Prevotella stercorea, Firmicutes bacterium CAG 110, Bacteroides salyersiae, Lactococcus petauri, Streptococcus cristatus, Actinomyces sp. HMSC035G02, and Streptococcus viridans. Widespread functional dysbiosis was detected across multiple biological levels in patients with WD, with significant correlations identified between these microbial alterations and clinical indicators. Significant disruptions were identified in key metabolic pathways, including the Pentose Phosphate Pathway, Pyruvate Metabolism, and Starch and Sucrose Metabolism, which were associated with the dysregulation of carbohydrate-active enzymes (CAZymes). These alterations showed significant correlations with clinical markers of liver dysfunction (e.g., procollagen III N-terminal peptide PIIINP, aspartate transaminase/alanine transaminase AST/ALT). A total of 54 virulence factor (VF) genes exhibited differential abundance in WD, with 36 genes depleted and 18 enriched. Notably, these included colibactin genes (clbB, clbH) from Escherichia coli and type IV secretion system genes (aec19, pilB). These VFs were significantly associated with indicators of liver function (e.g., bilirubin levels) and coagulation abnormalities. Among the detected antibiotic resistance genes (ARGs), 21 exhibited disease-specific patterns in WD, notably tetQ (encoding tetracycline resistance), ErmB (conferring macrolide resistance), and cfxA6 (mediating cephamycin resistance). Furthermore, ARG profiles were associated with Bifidobacterium enrichment and showed significant correlations with lipid metabolism markers [e.g., triglycerides (TG), high-density lipoprotein cholesterol (HDL-C)]. Critically, we identified significant enrichment of 60 functional mobile genetic elements (MGEs) in WD, spanning categories involved in DNA replication/repair, phage activity, and conjugative transfer, indicating heightened genomic plasticity and horizontal gene transfer potential. Strikingly, correlation network analysis revealed strong and specific co-occurrence between key ARGs (e.g., ErmX) and defined suites of MGEs, suggesting MGE-facilitated dissemination of resistance determinants.
CONCLUSION: Wilson's disease (WD) patients exhibit significant alterations in gut microbial community structure and functional dysbiosis, wherein the enrichment of virulence genes (such as colibactin genes clbB/clbH) and the specific antibiotic resistance genes (such as tetQ and ErmB), and the activation of mobile genetic elements are closely associated with clinical indicators including liver function impairment, coagulation abnormalities, and lipid metabolism disorders.
Additional Links: PMID-41623619
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41623619,
year = {2025},
author = {Wei, T and Qian, N and Wang, H and Song, Y and Wang, W and Li, Y and Zhao, Z and Xu, F and Yang, W},
title = {Wilson's disease-associated gut dysbiosis: novel insights into microbial functional alterations, virulence changes, and resistance markers.},
journal = {Frontiers in microbiology},
volume = {16},
number = {},
pages = {1714276},
pmid = {41623619},
issn = {1664-302X},
abstract = {BACKGROUND: Although the gut microbiota is associated with a variety of metabolic, inflammatory, and neurological disorders through microbial dysbiosis, current studies on the gut microbiota in Wilson's disease (WD) remain limited. Critical gaps exist in understanding the roles of key functional microbial factors in WD pathogenesis, which hinders the acquisition of mechanistic insights into this disease.
OBJECTIVE: This study aims to characterize alterations in the gut microbiome associated with WD, with a particular emphasis on virulence factors (VFs) and antibiotic resistance genes (ARGs), as well as functional mobile genetic elements (MGEs), in order to elucidate their potential roles in disease progression and clinical manifestations.
METHODS: We analyzed fecal samples from 37 patients with WD and 33 healthy controls (HCs) using metagenomic sequencing, with a specific focus on examining virulence gene profiles and antibiotic resistance patterns and MGE composition in relation to liver function markers.
RESULTS: Beta diversity analysis revealed significant differences in the gut microbial community structure between patients with WD and HCs, and a distinct set of microbial taxa was identified that showed significant associations with clinical indicators. A gut microbial co-occurrence network identified key species playing central roles in the microbial community structure, including Prevotella stercorea, Firmicutes bacterium CAG 110, Bacteroides salyersiae, Lactococcus petauri, Streptococcus cristatus, Actinomyces sp. HMSC035G02, and Streptococcus viridans. Widespread functional dysbiosis was detected across multiple biological levels in patients with WD, with significant correlations identified between these microbial alterations and clinical indicators. Significant disruptions were identified in key metabolic pathways, including the Pentose Phosphate Pathway, Pyruvate Metabolism, and Starch and Sucrose Metabolism, which were associated with the dysregulation of carbohydrate-active enzymes (CAZymes). These alterations showed significant correlations with clinical markers of liver dysfunction (e.g., procollagen III N-terminal peptide PIIINP, aspartate transaminase/alanine transaminase AST/ALT). A total of 54 virulence factor (VF) genes exhibited differential abundance in WD, with 36 genes depleted and 18 enriched. Notably, these included colibactin genes (clbB, clbH) from Escherichia coli and type IV secretion system genes (aec19, pilB). These VFs were significantly associated with indicators of liver function (e.g., bilirubin levels) and coagulation abnormalities. Among the detected antibiotic resistance genes (ARGs), 21 exhibited disease-specific patterns in WD, notably tetQ (encoding tetracycline resistance), ErmB (conferring macrolide resistance), and cfxA6 (mediating cephamycin resistance). Furthermore, ARG profiles were associated with Bifidobacterium enrichment and showed significant correlations with lipid metabolism markers [e.g., triglycerides (TG), high-density lipoprotein cholesterol (HDL-C)]. Critically, we identified significant enrichment of 60 functional mobile genetic elements (MGEs) in WD, spanning categories involved in DNA replication/repair, phage activity, and conjugative transfer, indicating heightened genomic plasticity and horizontal gene transfer potential. Strikingly, correlation network analysis revealed strong and specific co-occurrence between key ARGs (e.g., ErmX) and defined suites of MGEs, suggesting MGE-facilitated dissemination of resistance determinants.
CONCLUSION: Wilson's disease (WD) patients exhibit significant alterations in gut microbial community structure and functional dysbiosis, wherein the enrichment of virulence genes (such as colibactin genes clbB/clbH) and the specific antibiotic resistance genes (such as tetQ and ErmB), and the activation of mobile genetic elements are closely associated with clinical indicators including liver function impairment, coagulation abnormalities, and lipid metabolism disorders.},
}
RevDate: 2026-02-02
Engineering next-generation crops through CRISPR-mediated horizontal gene transfer.
The New phytologist [Epub ahead of print].
Crops increasingly face overlapping stresses such as heat, drought, salinity, and pathogens that conventional breeding or genome editing rarely overcome in combination. To address this, we propose CRISPR-enabled horizontal gene transfer (CRISPR-HGT) as a programmable framework that recreates the evolutionary process by which plants historically acquired adaptive microbial genes. Microbial genes, refined under extreme environments, provide a naturally preadapted resource for multi-trait resilience. By integrating tools such as Cas12a, CasΦ, RNA-targeting, and dCas-based epigenome editors with AI-guided microbial gene discovery, CRISPR-HGT enables modular and inducible stress regulation. This approach shifts genome editing from allelic modification to evolution-guided design. We outline a conceptual pipeline spanning microbial gene mining to adaptive field deployment, highlighting the ecological, biosafety, and regulatory dimensions, from the European Union's cautious oversight to the UK's product-based framework. CRISPR-HGT thus introduces an evolution-informed paradigm for engineering crops that anticipate stress and sustain yield under climate uncertainty.
Additional Links: PMID-41622828
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41622828,
year = {2026},
author = {Sen, MK and Roy, A and Varshney, RK and Chakraborty, A},
title = {Engineering next-generation crops through CRISPR-mediated horizontal gene transfer.},
journal = {The New phytologist},
volume = {},
number = {},
pages = {},
doi = {10.1111/nph.70951},
pmid = {41622828},
issn = {1469-8137},
abstract = {Crops increasingly face overlapping stresses such as heat, drought, salinity, and pathogens that conventional breeding or genome editing rarely overcome in combination. To address this, we propose CRISPR-enabled horizontal gene transfer (CRISPR-HGT) as a programmable framework that recreates the evolutionary process by which plants historically acquired adaptive microbial genes. Microbial genes, refined under extreme environments, provide a naturally preadapted resource for multi-trait resilience. By integrating tools such as Cas12a, CasΦ, RNA-targeting, and dCas-based epigenome editors with AI-guided microbial gene discovery, CRISPR-HGT enables modular and inducible stress regulation. This approach shifts genome editing from allelic modification to evolution-guided design. We outline a conceptual pipeline spanning microbial gene mining to adaptive field deployment, highlighting the ecological, biosafety, and regulatory dimensions, from the European Union's cautious oversight to the UK's product-based framework. CRISPR-HGT thus introduces an evolution-informed paradigm for engineering crops that anticipate stress and sustain yield under climate uncertainty.},
}
RevDate: 2026-02-01
CmpDate: 2026-02-01
Reassessing viral origins and evolutionary placement in the tree of life.
Antonie van Leeuwenhoek, 119(2):45.
The quest to fit all cellular beings in one picture frame as the universal Tree of Life (ToL) has always been a daunting task for evolutionary biologists. Over the decades, ToL has emerged from a dichotomous topology to its present form with three domains; bacteria, archaea and eukarya. But this phylogenetic placement is also questionable due to the miscellaneous nature of certain housekeeping genes, horizontal gene transfers (HGT), and also due to incomplete pathways of pathogenic organisms. Furthermore, the ambiguous nature of viruses has always puzzled researchers about their placement in ToL. Despite the multiple attempts, lack of common genes, and their coevolution with host systems, placement of viruses has always been controversial and has often yielded scattered phylogeny among themselves. Recent discoveries-especially of giant viruses sharing genes with cellular domains-offer fresh insights that support the inclusion of viruses in the ToL framework. By focusing on the RNA polymerase subunit β (RpoB) gene, a conserved marker across bacteria, archaea, eukarya, and giant viruses, this study reconstructs phylogenies that reveal giant viruses clustering closely with eukaryotes, suggesting viruses may occupy a distinct yet integral position in the evolutionary landscape. Though perfect declaration of viruses as fourth domain is still dubious, their placement in ToL is as important as any other cellular organism.
Additional Links: PMID-41622378
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41622378,
year = {2026},
author = {Jit, S and Kaur, J and Jain, A and Raina, D and Lal, R and Verma, M},
title = {Reassessing viral origins and evolutionary placement in the tree of life.},
journal = {Antonie van Leeuwenhoek},
volume = {119},
number = {2},
pages = {45},
pmid = {41622378},
issn = {1572-9699},
mesh = {*Phylogeny ; Archaea/genetics/virology/classification ; Bacteria/genetics/virology/classification ; *Viruses/genetics/classification ; *Evolution, Molecular ; Gene Transfer, Horizontal ; Eukaryota/genetics/classification ; *Biological Evolution ; Giant Viruses/genetics/classification ; },
abstract = {The quest to fit all cellular beings in one picture frame as the universal Tree of Life (ToL) has always been a daunting task for evolutionary biologists. Over the decades, ToL has emerged from a dichotomous topology to its present form with three domains; bacteria, archaea and eukarya. But this phylogenetic placement is also questionable due to the miscellaneous nature of certain housekeeping genes, horizontal gene transfers (HGT), and also due to incomplete pathways of pathogenic organisms. Furthermore, the ambiguous nature of viruses has always puzzled researchers about their placement in ToL. Despite the multiple attempts, lack of common genes, and their coevolution with host systems, placement of viruses has always been controversial and has often yielded scattered phylogeny among themselves. Recent discoveries-especially of giant viruses sharing genes with cellular domains-offer fresh insights that support the inclusion of viruses in the ToL framework. By focusing on the RNA polymerase subunit β (RpoB) gene, a conserved marker across bacteria, archaea, eukarya, and giant viruses, this study reconstructs phylogenies that reveal giant viruses clustering closely with eukaryotes, suggesting viruses may occupy a distinct yet integral position in the evolutionary landscape. Though perfect declaration of viruses as fourth domain is still dubious, their placement in ToL is as important as any other cellular organism.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Phylogeny
Archaea/genetics/virology/classification
Bacteria/genetics/virology/classification
*Viruses/genetics/classification
*Evolution, Molecular
Gene Transfer, Horizontal
Eukaryota/genetics/classification
*Biological Evolution
Giant Viruses/genetics/classification
RevDate: 2026-02-02
CmpDate: 2026-02-02
Horizontal transfer of ΦHKU.vir and its role in the evolution of acapsular emm89 group A Streptococcus in Taiwan.
Infection, 54(1):191-201.
INTRODUCTION: The global resurgence of scarlet fever and invasive group A Streptococcus (GAS) infections has been noted over the past decade. In East Asia, specifically in Hong Kong and China, emm12 isolates that acquired prophage ΦHKU.vir, which carried SSA, SpeC, and Spd1 exotoxins, were over-presented in scarlet fever cases. The prevalence of ssa-positive emm12 isolates was increased significantly in Taiwan; however, it remains unclear whether this increase is mediated by the horizontal transfer of ΦHKU.vir homologs or the expansion of Hong Kong scarlet fever-associated emm12 isolates.
MATERIALS AND METHODS: This study included 240 non-emm1 isolates in Taiwan during 2009-2023. The genome and prophage sequences of clinical isolates were analyzed by whole genome sequencing.
RESULTS: The prophages carried ssa, speC, and spd1 in Taiwan emm12 isolates shared high nucleotide sequence identity with ΦHKU.vir. All analyzed emm12 isolates in Taiwan were phylogenetically closely related to Hong Kong emm12 isolates, suggesting that Taiwan ssa-positive emm12 isolates shared a common origin with those from Hong Kong. This study further identified ΦHKU.vir homologs in emm90 and acapsular emm89 isolates. Although the acquisition of ΦHKU.vir is related to the expansion of emm12 isolates in Hong Kong, this study suggests that the prophage exotoxin SSA did not have significant roles in enhancing bacterial cytotoxicity and intracelluar survival of the acapsular emm89 strains.
CONCLUSIONS: The acquisition of prophages is important for the evolution of GAS. Monitoring the expansion of ΦHKU.vir in non-emm1/emm12 isolates is essential, as studying its impact on GAS pathogenicity will help in preventing and controlling GAS infections.
Additional Links: PMID-40996673
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid40996673,
year = {2026},
author = {Chiang-Ni, C and Lee, CC and Chi, CY and Lin, MC and Hsu, YC and Pan, MH and Hsu, CY and Chiu, CH},
title = {Horizontal transfer of ΦHKU.vir and its role in the evolution of acapsular emm89 group A Streptococcus in Taiwan.},
journal = {Infection},
volume = {54},
number = {1},
pages = {191-201},
pmid = {40996673},
issn = {1439-0973},
support = {113-2320-B-182-008 , 114-2320-B-182-022-MY3//National Science and Technology Council/ ; CMRPD1P0251, BMRPD19//Chang Gung Memorial Hospital, Linkou/ ; },
mesh = {Taiwan/epidemiology ; *Streptococcus pyogenes/genetics/virology/isolation & purification/classification ; *Gene Transfer, Horizontal ; Humans ; *Prophages/genetics ; *Streptococcal Infections/microbiology/epidemiology ; Phylogeny ; Whole Genome Sequencing ; Scarlet Fever/microbiology/epidemiology ; },
abstract = {INTRODUCTION: The global resurgence of scarlet fever and invasive group A Streptococcus (GAS) infections has been noted over the past decade. In East Asia, specifically in Hong Kong and China, emm12 isolates that acquired prophage ΦHKU.vir, which carried SSA, SpeC, and Spd1 exotoxins, were over-presented in scarlet fever cases. The prevalence of ssa-positive emm12 isolates was increased significantly in Taiwan; however, it remains unclear whether this increase is mediated by the horizontal transfer of ΦHKU.vir homologs or the expansion of Hong Kong scarlet fever-associated emm12 isolates.
MATERIALS AND METHODS: This study included 240 non-emm1 isolates in Taiwan during 2009-2023. The genome and prophage sequences of clinical isolates were analyzed by whole genome sequencing.
RESULTS: The prophages carried ssa, speC, and spd1 in Taiwan emm12 isolates shared high nucleotide sequence identity with ΦHKU.vir. All analyzed emm12 isolates in Taiwan were phylogenetically closely related to Hong Kong emm12 isolates, suggesting that Taiwan ssa-positive emm12 isolates shared a common origin with those from Hong Kong. This study further identified ΦHKU.vir homologs in emm90 and acapsular emm89 isolates. Although the acquisition of ΦHKU.vir is related to the expansion of emm12 isolates in Hong Kong, this study suggests that the prophage exotoxin SSA did not have significant roles in enhancing bacterial cytotoxicity and intracelluar survival of the acapsular emm89 strains.
CONCLUSIONS: The acquisition of prophages is important for the evolution of GAS. Monitoring the expansion of ΦHKU.vir in non-emm1/emm12 isolates is essential, as studying its impact on GAS pathogenicity will help in preventing and controlling GAS infections.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
Taiwan/epidemiology
*Streptococcus pyogenes/genetics/virology/isolation & purification/classification
*Gene Transfer, Horizontal
Humans
*Prophages/genetics
*Streptococcal Infections/microbiology/epidemiology
Phylogeny
Whole Genome Sequencing
Scarlet Fever/microbiology/epidemiology
RevDate: 2026-02-01
From Interface to Cell: The Complex Interaction and Transfer Process Coupling Mechanism between Microplastics and Antibiotic Resistance Genes.
Environmental science & technology [Epub ahead of print].
Microplastic-phase interfaces (MPPIs) were established as critical vectors for accelerating antibiotic resistance gene (ARG) dissemination. Through integrated anaerobic/aerobic wastewater treatment system experiments combined with physicochemical characterization, metagenomic sequencing, and molecular dynamics simulations (MD), we elucidated MP-ARG interaction mechanisms from the interfacial to the cellular scale. Polyethylene terephthalate (PET), polyethylene (PE), and polypropylene (PP) MPPIs underwent significant aging during 60 days of exposure, resulting in elemental enrichment (C/O/P), the formation of C═C/C-H/C-O/C-OH functional groups, and elevated oxidation. These transformations enhanced extracellular polymeric substance production (184.81 mg/g MLSS) and selectively enriched antibiotic-resistant bacteria, ARGs, and mobile genetic elements (MGEs), promoting horizontal gene transfer. XDLVO theory revealed spontaneous microbial adhesion (ΔGadh = -23.63 mJ/m[2]) driven by Lifshitz-van der Waals (LW) and acid-base interactions. MD demonstrated direct MP penetration into the membrane via dominant LW forces (-1200 kJ/mol) and increased permeability. Concurrently, compared with sewage water (SW), MPPIs induced a 2.06-fold overproduction of reactive oxygen species, which upregulated genes encoding efflux pumps (acrF, 3.2-fold), outer membrane porins (OmpF, 4.1-fold), and conjugative transfer genes (traF, 3.8-fold). Material-specific (PET > PE > PP) and oxygen-driven redox mechanisms governed ARG dissemination: aerobic conditions favored radical-driven oxidation and MGE entrapment, whereas anaerobic systems enhanced hydrophobic adhesion.
Additional Links: PMID-41620987
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41620987,
year = {2026},
author = {Tian, H and Liu, J and Li, L and Ge, J},
title = {From Interface to Cell: The Complex Interaction and Transfer Process Coupling Mechanism between Microplastics and Antibiotic Resistance Genes.},
journal = {Environmental science & technology},
volume = {},
number = {},
pages = {},
doi = {10.1021/acs.est.5c11841},
pmid = {41620987},
issn = {1520-5851},
abstract = {Microplastic-phase interfaces (MPPIs) were established as critical vectors for accelerating antibiotic resistance gene (ARG) dissemination. Through integrated anaerobic/aerobic wastewater treatment system experiments combined with physicochemical characterization, metagenomic sequencing, and molecular dynamics simulations (MD), we elucidated MP-ARG interaction mechanisms from the interfacial to the cellular scale. Polyethylene terephthalate (PET), polyethylene (PE), and polypropylene (PP) MPPIs underwent significant aging during 60 days of exposure, resulting in elemental enrichment (C/O/P), the formation of C═C/C-H/C-O/C-OH functional groups, and elevated oxidation. These transformations enhanced extracellular polymeric substance production (184.81 mg/g MLSS) and selectively enriched antibiotic-resistant bacteria, ARGs, and mobile genetic elements (MGEs), promoting horizontal gene transfer. XDLVO theory revealed spontaneous microbial adhesion (ΔGadh = -23.63 mJ/m[2]) driven by Lifshitz-van der Waals (LW) and acid-base interactions. MD demonstrated direct MP penetration into the membrane via dominant LW forces (-1200 kJ/mol) and increased permeability. Concurrently, compared with sewage water (SW), MPPIs induced a 2.06-fold overproduction of reactive oxygen species, which upregulated genes encoding efflux pumps (acrF, 3.2-fold), outer membrane porins (OmpF, 4.1-fold), and conjugative transfer genes (traF, 3.8-fold). Material-specific (PET > PE > PP) and oxygen-driven redox mechanisms governed ARG dissemination: aerobic conditions favored radical-driven oxidation and MGE entrapment, whereas anaerobic systems enhanced hydrophobic adhesion.},
}
RevDate: 2026-01-31
Horizontal transfer of post-translational modifiers brings evolutionary opportunity and challenges to a conserved translation factor.
BMC biology pii:10.1186/s12915-026-02531-9 [Epub ahead of print].
BACKGROUND: Horizontal gene transfer (HGT) is a major driver of microbial evolution, yet the influence of host cellular context on the integration and functionality of transferred genes remains underexplored. In this study, we investigate how host background impacts the horizontal acquisition of post-translational modification (PTM) machinery. Here, we use heterologous expression of the highly conserved and frequently horizontally transferred translational elongation factor P (EF-P) from diverse species in Escherichia coli as a model. EF-P has a heterogenous relationship with PTMs; three characterized variants each undergo distinct PTM pathways, while others function effectively without any modification.
RESULTS: We demonstrate that EF-P from Deinococcus radiodurans, Geoalkalibacter ferrihydriticus, and Nitrosomonas communis can complement an EF-P knockout in E. coli without requiring any PTM, suggesting they may represent new examples of unmodified EF-P. We also found that the EF-P from the Thermotogota Mesotoga prima is post-translationally modified in an off-target reaction by the rhamnosylation enzyme EarP, thus interfering with its functionality. Conversely, we saw that rhamnosylation by EarP does not impact the function of the EF-P-like protein EfpL.
CONCLUSIONS: Our findings highlight that PTM systems introduced via HGT can have varied effects on host proteins. We found that different EF-P variants are impacted in different ways by off-target rhamnosylation. While some of these off-target reactions may present opportunities to develop novel, catalytically active PTMs, others are detrimental to the function of the modified EF-P. Our results emphasize the complexity of gene integration and functional compatibility in foreign genomic contexts.
Additional Links: PMID-41618333
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41618333,
year = {2026},
author = {Brewer, TE and Kielkowski, P and Stritzel, J and Meier-Rosar, F and Schlundt, A and Lassak, J},
title = {Horizontal transfer of post-translational modifiers brings evolutionary opportunity and challenges to a conserved translation factor.},
journal = {BMC biology},
volume = {},
number = {},
pages = {},
doi = {10.1186/s12915-026-02531-9},
pmid = {41618333},
issn = {1741-7007},
support = {210991//Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung/ ; SFB1309 - 325871075//Deutsche Forschungsgemeinschaft/ ; LA 3658/1-3//Deutsche Forschungsgemeinschaft/ ; },
abstract = {BACKGROUND: Horizontal gene transfer (HGT) is a major driver of microbial evolution, yet the influence of host cellular context on the integration and functionality of transferred genes remains underexplored. In this study, we investigate how host background impacts the horizontal acquisition of post-translational modification (PTM) machinery. Here, we use heterologous expression of the highly conserved and frequently horizontally transferred translational elongation factor P (EF-P) from diverse species in Escherichia coli as a model. EF-P has a heterogenous relationship with PTMs; three characterized variants each undergo distinct PTM pathways, while others function effectively without any modification.
RESULTS: We demonstrate that EF-P from Deinococcus radiodurans, Geoalkalibacter ferrihydriticus, and Nitrosomonas communis can complement an EF-P knockout in E. coli without requiring any PTM, suggesting they may represent new examples of unmodified EF-P. We also found that the EF-P from the Thermotogota Mesotoga prima is post-translationally modified in an off-target reaction by the rhamnosylation enzyme EarP, thus interfering with its functionality. Conversely, we saw that rhamnosylation by EarP does not impact the function of the EF-P-like protein EfpL.
CONCLUSIONS: Our findings highlight that PTM systems introduced via HGT can have varied effects on host proteins. We found that different EF-P variants are impacted in different ways by off-target rhamnosylation. While some of these off-target reactions may present opportunities to develop novel, catalytically active PTMs, others are detrimental to the function of the modified EF-P. Our results emphasize the complexity of gene integration and functional compatibility in foreign genomic contexts.},
}
RevDate: 2026-02-01
Plant recognition by Trichoderma Harzianum elicits upregulation of a novel secondary metabolite cluster required for colonization.
Scientific reports, 16(1):3945.
UNLABELLED: Trichoderma harzianum is a filamentous ascomycete frequently applied as biocontrol agent in agriculture. While mycoparasitism and antagonism of Trichoderma spp. against fungal pathogens are well known, early fungal responses to the presence of a plant await broader investigation. Analyzing early stages of plant-fungus communication we show that T. harzianum B97 chemotropically responds to a plant extract and that both plant and fungus alter secondary metabolite secretion upon recognition. We developed a strategy for omics-analysis simulating conditions of early plant recognition eliciting a chemotropic response in the fungus and found 102 genes to be differentially regulated, including nitrate and nitrite reductases. Additionally, the previously uncharacterized Plant Communication Associated (PCA) gene cluster was strongly induced upon recognition of the plant, comprises a palindromic DNA motif and was essential for plant colonization. The PCA-cluster is only present in the Harzianum clade of Trichoderma and closely related to a homologous cluster in Metarhizium spp. Horizontal gene transfer (HGT) was detected for PCA-cluster genes by plants, while the cluster in T. harzianum is likely under balancing or positive selection. Hence, the PCA-cluster mediates early fungus-plant chemical communication and may be responsible for the high potential of T. harzianum and closely related species for biocontrol applications.
SUPPLEMENTARY INFORMATION: The online version contains supplementary material available at 10.1038/s41598-025-33935-2.
Additional Links: PMID-41559304
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41559304,
year = {2026},
author = {Schalamun, M and Li, G and Hinterdobler, W and Großkinsky, DK and Compant, S and Dreux-Zigha, A and Gerke, J and Cox, R and Schmoll, M},
title = {Plant recognition by Trichoderma Harzianum elicits upregulation of a novel secondary metabolite cluster required for colonization.},
journal = {Scientific reports},
volume = {16},
number = {1},
pages = {3945},
pmid = {41559304},
issn = {2045-2322},
abstract = {UNLABELLED: Trichoderma harzianum is a filamentous ascomycete frequently applied as biocontrol agent in agriculture. While mycoparasitism and antagonism of Trichoderma spp. against fungal pathogens are well known, early fungal responses to the presence of a plant await broader investigation. Analyzing early stages of plant-fungus communication we show that T. harzianum B97 chemotropically responds to a plant extract and that both plant and fungus alter secondary metabolite secretion upon recognition. We developed a strategy for omics-analysis simulating conditions of early plant recognition eliciting a chemotropic response in the fungus and found 102 genes to be differentially regulated, including nitrate and nitrite reductases. Additionally, the previously uncharacterized Plant Communication Associated (PCA) gene cluster was strongly induced upon recognition of the plant, comprises a palindromic DNA motif and was essential for plant colonization. The PCA-cluster is only present in the Harzianum clade of Trichoderma and closely related to a homologous cluster in Metarhizium spp. Horizontal gene transfer (HGT) was detected for PCA-cluster genes by plants, while the cluster in T. harzianum is likely under balancing or positive selection. Hence, the PCA-cluster mediates early fungus-plant chemical communication and may be responsible for the high potential of T. harzianum and closely related species for biocontrol applications.
SUPPLEMENTARY INFORMATION: The online version contains supplementary material available at 10.1038/s41598-025-33935-2.},
}
RevDate: 2026-01-31
Tiny packages, big potential: bacterial membrane vesicles in vaccinology.
Microbial cell factories, 25(1):31.
Bacterial membrane vesicles (BMVs) are nanoscale, bilayered proteolipid structures secreted by both Gram-negative and Gram-positive bacteria. Initially considered cellular debris, BMVs are now recognized as evolutionarily conserved entities with critical roles in bacterial communication, immune modulation, virulence factor delivery, and horizontal gene transfer. Their structural and functional resemblance to eukaryotic extracellular vesicles has fueled growing interest in their use as versatile vaccine platforms. Licensed meningococcal OMV vaccines established proof-of-concept for their safety and immunogenicity, and ongoing studies are extending applications to enteric pathogens and viral infections. Recent advances in genetic engineering, glycoengineering, and modular antigen display systems have enabled the design of “plug-and-play” BMVs with reduced reactogenicity and enhanced protective efficacy. In parallel, innovations in bioprocessing and formulation technologies are improving scalability, stability, and delivery, including mucosal routes. This review highlights the immunological properties, translational potential, and key challenges of BMV-based vaccines, with an emphasis on strategies to optimize safety, antigen specificity, and manufacturing for next-generation vaccine development.
Additional Links: PMID-41469689
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41469689,
year = {2025},
author = {Varol, A and Aydın, Ş and Adıgüzel, A and Özdemir, S},
title = {Tiny packages, big potential: bacterial membrane vesicles in vaccinology.},
journal = {Microbial cell factories},
volume = {25},
number = {1},
pages = {31},
pmid = {41469689},
issn = {1475-2859},
abstract = {Bacterial membrane vesicles (BMVs) are nanoscale, bilayered proteolipid structures secreted by both Gram-negative and Gram-positive bacteria. Initially considered cellular debris, BMVs are now recognized as evolutionarily conserved entities with critical roles in bacterial communication, immune modulation, virulence factor delivery, and horizontal gene transfer. Their structural and functional resemblance to eukaryotic extracellular vesicles has fueled growing interest in their use as versatile vaccine platforms. Licensed meningococcal OMV vaccines established proof-of-concept for their safety and immunogenicity, and ongoing studies are extending applications to enteric pathogens and viral infections. Recent advances in genetic engineering, glycoengineering, and modular antigen display systems have enabled the design of “plug-and-play” BMVs with reduced reactogenicity and enhanced protective efficacy. In parallel, innovations in bioprocessing and formulation technologies are improving scalability, stability, and delivery, including mucosal routes. This review highlights the immunological properties, translational potential, and key challenges of BMV-based vaccines, with an emphasis on strategies to optimize safety, antigen specificity, and manufacturing for next-generation vaccine development.},
}
RevDate: 2026-01-30
Human activities and horizontal gene transfer shape the resistome landscapes of non-human primates.
Journal of hazardous materials, 504:141276 pii:S0304-3894(26)00254-2 [Epub ahead of print].
Antibiotic resistance represents a growing threat to human, animal, and ecosystem health, yet its dynamics in wildlife remain poorly understood. We conducted a systematic analysis of the gut resistomes in non-human primates (NHPs) and environmental soils in Guizhou Province, China, a biodiversity hotspot. Metagenomic analyses reveal that human activities and horizontal gene transfer (HGT) influence primate resistome landscapes and enhance their dissemination potential. A total of 1927 antibiotic resistance ontologies (AROs) distributed across 1477 species-level genome bins (SGBs), providing a comprehensive genomic catalog of the NHPs resistome. Bacterial genera such as Pseudomonas, Stenotrophomonas, and Comamonas drive ARG mobilization, with a core subset of ARGs that reliably predict overall resistance burdens. Notably, widely distributed primate species, with large habitat ranges and frequent interspecies interactions exhibit the most potential for ARG dissemination. Ecological modeling identifies current and future hotspot regions requiring prioritized monitoring amid ongoing human disturbance and climate change. These findings provide a molecular-indicator-based framework for environmental antibiotic resistance (AR) monitoring and conservation strategies for endangered species. Despite limitations in temporal and spatial coverage, our study highlights the need to integrate wildlife, particularly NHPs, as sentinel species into "One Health" AR surveillance and policy. This approach will strengthen our understanding of ARG transmission dynamics and their long-term impacts on host adaptation, ecosystem stability, and public health.
Additional Links: PMID-41616624
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41616624,
year = {2026},
author = {Sun, Y and Zhang, M and Teng, Y and Yin, Y and Ran, J and Su, H and Li, H and Huang, X and Long, Z and Sun, X and Pan, H and Wang, X and Li, M},
title = {Human activities and horizontal gene transfer shape the resistome landscapes of non-human primates.},
journal = {Journal of hazardous materials},
volume = {504},
number = {},
pages = {141276},
doi = {10.1016/j.jhazmat.2026.141276},
pmid = {41616624},
issn = {1873-3336},
abstract = {Antibiotic resistance represents a growing threat to human, animal, and ecosystem health, yet its dynamics in wildlife remain poorly understood. We conducted a systematic analysis of the gut resistomes in non-human primates (NHPs) and environmental soils in Guizhou Province, China, a biodiversity hotspot. Metagenomic analyses reveal that human activities and horizontal gene transfer (HGT) influence primate resistome landscapes and enhance their dissemination potential. A total of 1927 antibiotic resistance ontologies (AROs) distributed across 1477 species-level genome bins (SGBs), providing a comprehensive genomic catalog of the NHPs resistome. Bacterial genera such as Pseudomonas, Stenotrophomonas, and Comamonas drive ARG mobilization, with a core subset of ARGs that reliably predict overall resistance burdens. Notably, widely distributed primate species, with large habitat ranges and frequent interspecies interactions exhibit the most potential for ARG dissemination. Ecological modeling identifies current and future hotspot regions requiring prioritized monitoring amid ongoing human disturbance and climate change. These findings provide a molecular-indicator-based framework for environmental antibiotic resistance (AR) monitoring and conservation strategies for endangered species. Despite limitations in temporal and spatial coverage, our study highlights the need to integrate wildlife, particularly NHPs, as sentinel species into "One Health" AR surveillance and policy. This approach will strengthen our understanding of ARG transmission dynamics and their long-term impacts on host adaptation, ecosystem stability, and public health.},
}
RevDate: 2026-01-30
Long-read sequencing for bacterial plasmid analysis: a brief overview.
FEMS microbiology letters pii:8445395 [Epub ahead of print].
Whole-genome sequencing (WGS) has transformed microbial genomics since the first bacterial genome was published in 1995. Advances in sequencing technology, together with decreasing costs, now enable high-resolution investigation of bacterial pathogens for epidemiological surveillance and infection control. A major breakthrough has been the advent of third-generation long-read sequencing (LRS) platforms, such as Pacific Biosciences and Oxford Nanopore Technologies, which overcome the limitations of short-read sequencing (SRS) by producing long continuous reads. LRS facilitates accurate de novo genome assembly, resolution of repetitive and structurally complex regions, and precise characterization of plasmids and other mobile genetic elements (MGEs) that frequently harbor antimicrobial resistance genes (ARGs). A particular strength of LRS lies in its ability to reveal the complete genomic architecture of ARGs, including their localization, copy number, and surrounding genetic environment. Such contextual information is essential, since e.g. the interpretation of antimicrobial resistance (AMR) depends not only on the presence of specific genes but also on their structural organization, mobility potential, and genomic integration. By contrast, LRS provides a reliable foundation for understanding AMR evolution and dissemination through both clonal expansion and horizontal gene transfer. Recent developments in bioinformatics, including dedicated tools for plasmid reconstruction, typing, and annotation, further enhance the analytical value of LRS and hybrid approaches. Beyond isolate-level analyses, LRS enables plasmid surveillance and the tracing of ARG transmission across strains, hosts, and healthcare settings. This review sets out to give readers a brief overview of LRS technology and its capabilities and outlines current approaches and tools to analyze bacterial plasmids.
Additional Links: PMID-41614961
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41614961,
year = {2026},
author = {van Almsick, V and Sobkowiak, A and Schwierzeck, V},
title = {Long-read sequencing for bacterial plasmid analysis: a brief overview.},
journal = {FEMS microbiology letters},
volume = {},
number = {},
pages = {},
doi = {10.1093/femsle/fnag014},
pmid = {41614961},
issn = {1574-6968},
abstract = {Whole-genome sequencing (WGS) has transformed microbial genomics since the first bacterial genome was published in 1995. Advances in sequencing technology, together with decreasing costs, now enable high-resolution investigation of bacterial pathogens for epidemiological surveillance and infection control. A major breakthrough has been the advent of third-generation long-read sequencing (LRS) platforms, such as Pacific Biosciences and Oxford Nanopore Technologies, which overcome the limitations of short-read sequencing (SRS) by producing long continuous reads. LRS facilitates accurate de novo genome assembly, resolution of repetitive and structurally complex regions, and precise characterization of plasmids and other mobile genetic elements (MGEs) that frequently harbor antimicrobial resistance genes (ARGs). A particular strength of LRS lies in its ability to reveal the complete genomic architecture of ARGs, including their localization, copy number, and surrounding genetic environment. Such contextual information is essential, since e.g. the interpretation of antimicrobial resistance (AMR) depends not only on the presence of specific genes but also on their structural organization, mobility potential, and genomic integration. By contrast, LRS provides a reliable foundation for understanding AMR evolution and dissemination through both clonal expansion and horizontal gene transfer. Recent developments in bioinformatics, including dedicated tools for plasmid reconstruction, typing, and annotation, further enhance the analytical value of LRS and hybrid approaches. Beyond isolate-level analyses, LRS enables plasmid surveillance and the tracing of ARG transmission across strains, hosts, and healthcare settings. This review sets out to give readers a brief overview of LRS technology and its capabilities and outlines current approaches and tools to analyze bacterial plasmids.},
}
RevDate: 2026-01-30
CmpDate: 2026-01-30
Prevalence, characteristics, and plasmid dynamics of mcr-1 positive Enterobacteriaceae in Hainan, China: a preliminary genomic investigation.
Frontiers in microbiology, 16:1689159.
INTRODUCTION: The global spread of the plasmid-mediated colistin resistance gene mcr-1 poses a serious threat to public health. This study aimed to conduct a preliminary characterization of the epidemiology and genomic features of Enterobacteriaceae carrying the mcr-1 gene in a hospital setting in Hainan, China.
METHODS: A total of 2,700 Enterobacteriaceae strains, including 2,200 fecal samples and 500 respiratory, blood, and urine isolates, were collected from Haikou People's Hospital between October 2020 to September 2024. Specifically, the mcr-1 gene was screened by PCR. Antimicrobial susceptibility testing was performed with the VITEK 2 system. Four mcr-1 positive strains underwent whole-genome sequencing using Illumina and Nanopore platforms, which were combined with CARD, multilocus sequence typing (MLST), and plasmid analysis to elucidate resistance mechanisms.
RESULTS: The positivity rate for mcr-1 was 0.15% (4/2,700). All positive isolates were identified as Escherichia coli, with two strains originating from urine and two from fecal samples. Antimicrobial susceptibility testing showed that the urine isolates (C29 and C180) were extensively drug resistant (XDR). The fecal strain S321.4 was multidrug resistant (MDR), while S118.1 was sensitive. Patients with XDR/MDR strains had recent antibiotic exposure and invasive procedures. Whole-genome analysis revealed that MLST types of the strains were diverse (ST410, ST167, ST11165, ST1266), and mcr-1 was located on plasmids of IncI2 or IncX4 types. The IncI2 plasmid carried a complete conjugative operon. Plasmid C180_5 harbored bla CTX-M-199 through IS150, forming a multidrug resistance plasmid. Strain C29 exhibited a reduced colistin minimum inhibitory concentration (MIC) of 0.5 μg/mL due to disruption of mcr-1 by IS3, which likely impairs gene function. However, this requires further functional validation.
CONCLUSION: This preliminary study indicates a low prevalence of mcr-1 in our setting. However, the genomic identification of conjugative plasmids, including one carrying both mcr-1 and an extended-spectrum β-lactamase gene, highlights a tangible risk for horizontal co-transfer of resistance. The association of these isolates with healthcare exposures underscores the need for ongoing surveillance to monitor plasmid evolution in hospital ecosystems.
Additional Links: PMID-41614139
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41614139,
year = {2025},
author = {Wang, S and Han, X and Sheng, Y and Zhou, W and Huang, H and Wei, X},
title = {Prevalence, characteristics, and plasmid dynamics of mcr-1 positive Enterobacteriaceae in Hainan, China: a preliminary genomic investigation.},
journal = {Frontiers in microbiology},
volume = {16},
number = {},
pages = {1689159},
pmid = {41614139},
issn = {1664-302X},
abstract = {INTRODUCTION: The global spread of the plasmid-mediated colistin resistance gene mcr-1 poses a serious threat to public health. This study aimed to conduct a preliminary characterization of the epidemiology and genomic features of Enterobacteriaceae carrying the mcr-1 gene in a hospital setting in Hainan, China.
METHODS: A total of 2,700 Enterobacteriaceae strains, including 2,200 fecal samples and 500 respiratory, blood, and urine isolates, were collected from Haikou People's Hospital between October 2020 to September 2024. Specifically, the mcr-1 gene was screened by PCR. Antimicrobial susceptibility testing was performed with the VITEK 2 system. Four mcr-1 positive strains underwent whole-genome sequencing using Illumina and Nanopore platforms, which were combined with CARD, multilocus sequence typing (MLST), and plasmid analysis to elucidate resistance mechanisms.
RESULTS: The positivity rate for mcr-1 was 0.15% (4/2,700). All positive isolates were identified as Escherichia coli, with two strains originating from urine and two from fecal samples. Antimicrobial susceptibility testing showed that the urine isolates (C29 and C180) were extensively drug resistant (XDR). The fecal strain S321.4 was multidrug resistant (MDR), while S118.1 was sensitive. Patients with XDR/MDR strains had recent antibiotic exposure and invasive procedures. Whole-genome analysis revealed that MLST types of the strains were diverse (ST410, ST167, ST11165, ST1266), and mcr-1 was located on plasmids of IncI2 or IncX4 types. The IncI2 plasmid carried a complete conjugative operon. Plasmid C180_5 harbored bla CTX-M-199 through IS150, forming a multidrug resistance plasmid. Strain C29 exhibited a reduced colistin minimum inhibitory concentration (MIC) of 0.5 μg/mL due to disruption of mcr-1 by IS3, which likely impairs gene function. However, this requires further functional validation.
CONCLUSION: This preliminary study indicates a low prevalence of mcr-1 in our setting. However, the genomic identification of conjugative plasmids, including one carrying both mcr-1 and an extended-spectrum β-lactamase gene, highlights a tangible risk for horizontal co-transfer of resistance. The association of these isolates with healthcare exposures underscores the need for ongoing surveillance to monitor plasmid evolution in hospital ecosystems.},
}
RevDate: 2026-01-30
CmpDate: 2026-01-30
Bloodstream infection with NDM-1/5 Enterobacter cloacae complex in China: diverse STs, multi-virulence systems and carbapenem resistance.
Frontiers in cellular and infection microbiology, 15:1738317.
OBJECTIVES: To elucidate the molecular epidemiology, virulence repertoire and resistance gene characteristics of carbapenem-resistant Enterobacter cloacae complex (CRECC) in bloodstream infections (BSI), thereby providing evidence for precision therapy and infection control.
METHODS: We retrospectively collected 13 non-replicate CRECC-BSI isolates from January 2019 to December 2023 at a tertiary-care hospital in Shandong Province, China. Antimicrobial susceptibility was determined by broth microdilution; Illumina NovaSeq whole-genome sequencing was performed, and genomes were assembled with ABySS and GapCloser. ResFinder, VFDB, CGE and NCBI Pathogen Detection databases were used jointly to analyze resistance genes, virulence factors, plasmid replicons, MLST an SNP-based phylogenetic tree assessed inter-strain relatedness; while filter-mating assays determined the transferability of plasmids.
RESULTS: A total of 13 CRECC isolates yielded five sequence types (STs), with ST171 predominating (46.2%, 6/13); all carried bla NDM (bla NDM-1 in 9 isolates, bla NDM-5 in 4), along with AmpC, ESBLs, and aminoglycoside/quinolone resistance genes. The IncX3 plasmid replicon was most frequent (46.2%, 6/13), followed by IncHI2/HI2A (38.5%, 5/13). Each strain harbored adherence, biofilm formation, iron/manganese transport and T6SS virulence genes. Antimicrobial susceptibility testing revealed complete resistance among all isolates to cephalosporins, carbapenems and β-lactam/β-lactamase-inhibitor combinations, while amikacin, tigecycline and polymyxin B remained 100% susceptible. cgMLST revealed a polyclonal population structure. Conjugation assays demonstrated transfer of bla NDM-bearing plasmids to recipient Escherichia coli J53.
CONCLUSIONS: Our institutional CRECC-BSI is characterized by diverse sequence types, a complex plasmid profile and a high burden of virulence genes; ST171 is the dominant clone and bla NDM-1 the principal carbapenemase. Close surveillance of this high-risk lineage and of IncX3/IncHI2-mediated horizontal gene transfer is essential, together with strengthened infection-control and antimicrobial-stewardship measures.
Additional Links: PMID-41613598
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41613598,
year = {2025},
author = {Wang, X and Tian, Y and Zhang, Q and Jin, Y and Shao, C and Zhang, Z},
title = {Bloodstream infection with NDM-1/5 Enterobacter cloacae complex in China: diverse STs, multi-virulence systems and carbapenem resistance.},
journal = {Frontiers in cellular and infection microbiology},
volume = {15},
number = {},
pages = {1738317},
pmid = {41613598},
issn = {2235-2988},
mesh = {Humans ; *Enterobacter cloacae/genetics/drug effects/pathogenicity/isolation & purification/classification ; China/epidemiology ; *beta-Lactamases/genetics ; *Enterobacteriaceae Infections/microbiology/epidemiology ; Retrospective Studies ; Microbial Sensitivity Tests ; Plasmids/genetics ; Anti-Bacterial Agents/pharmacology ; Virulence Factors/genetics ; Multilocus Sequence Typing ; Carbapenems/pharmacology ; *Carbapenem-Resistant Enterobacteriaceae/genetics/drug effects/isolation & purification ; Phylogeny ; Molecular Epidemiology ; Virulence/genetics ; Whole Genome Sequencing ; *Bacteremia/microbiology/epidemiology ; *Drug Resistance, Multiple, Bacterial/genetics ; Male ; Bacterial Proteins/genetics ; Female ; Middle Aged ; Aged ; Tertiary Care Centers ; },
abstract = {OBJECTIVES: To elucidate the molecular epidemiology, virulence repertoire and resistance gene characteristics of carbapenem-resistant Enterobacter cloacae complex (CRECC) in bloodstream infections (BSI), thereby providing evidence for precision therapy and infection control.
METHODS: We retrospectively collected 13 non-replicate CRECC-BSI isolates from January 2019 to December 2023 at a tertiary-care hospital in Shandong Province, China. Antimicrobial susceptibility was determined by broth microdilution; Illumina NovaSeq whole-genome sequencing was performed, and genomes were assembled with ABySS and GapCloser. ResFinder, VFDB, CGE and NCBI Pathogen Detection databases were used jointly to analyze resistance genes, virulence factors, plasmid replicons, MLST an SNP-based phylogenetic tree assessed inter-strain relatedness; while filter-mating assays determined the transferability of plasmids.
RESULTS: A total of 13 CRECC isolates yielded five sequence types (STs), with ST171 predominating (46.2%, 6/13); all carried bla NDM (bla NDM-1 in 9 isolates, bla NDM-5 in 4), along with AmpC, ESBLs, and aminoglycoside/quinolone resistance genes. The IncX3 plasmid replicon was most frequent (46.2%, 6/13), followed by IncHI2/HI2A (38.5%, 5/13). Each strain harbored adherence, biofilm formation, iron/manganese transport and T6SS virulence genes. Antimicrobial susceptibility testing revealed complete resistance among all isolates to cephalosporins, carbapenems and β-lactam/β-lactamase-inhibitor combinations, while amikacin, tigecycline and polymyxin B remained 100% susceptible. cgMLST revealed a polyclonal population structure. Conjugation assays demonstrated transfer of bla NDM-bearing plasmids to recipient Escherichia coli J53.
CONCLUSIONS: Our institutional CRECC-BSI is characterized by diverse sequence types, a complex plasmid profile and a high burden of virulence genes; ST171 is the dominant clone and bla NDM-1 the principal carbapenemase. Close surveillance of this high-risk lineage and of IncX3/IncHI2-mediated horizontal gene transfer is essential, together with strengthened infection-control and antimicrobial-stewardship measures.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
Humans
*Enterobacter cloacae/genetics/drug effects/pathogenicity/isolation & purification/classification
China/epidemiology
*beta-Lactamases/genetics
*Enterobacteriaceae Infections/microbiology/epidemiology
Retrospective Studies
Microbial Sensitivity Tests
Plasmids/genetics
Anti-Bacterial Agents/pharmacology
Virulence Factors/genetics
Multilocus Sequence Typing
Carbapenems/pharmacology
*Carbapenem-Resistant Enterobacteriaceae/genetics/drug effects/isolation & purification
Phylogeny
Molecular Epidemiology
Virulence/genetics
Whole Genome Sequencing
*Bacteremia/microbiology/epidemiology
*Drug Resistance, Multiple, Bacterial/genetics
Male
Bacterial Proteins/genetics
Female
Middle Aged
Aged
Tertiary Care Centers
RevDate: 2026-01-30
Bacterial metabolic signatures in MASLD predicted through gene-centric studies in stool metagenomes.
BMC microbiology, 26(1):70.
BACKGROUND: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a multifactorial condition in which the gut microbiome (GM) plays a central role. However, taxonomic associations derived from 16S ribosomal RNA (rRNA) gene studies have yielded inconsistent results, likely due to limited resolution and functional redundancy across taxa. We aimed to identify robust, functionally relevant microbial markers of MASLD using metagenomics and gene-centric profiling.
METHODS: We analyzed 554 fecal metagenomes from three independent cohorts. Sequencing reads were quality-controlled and taxonomically profiled with multi-marker gene resolution. We quantified the abundance of over 50 target gene families involved in butyrate, methane, trimethylamine (TMA) and short-chain alcohol (SCAs, i.e., ethanol and propanol) metabolism. Their presence was also determined across complete GM genomes and plasmids.
RESULTS: Genes involved in butyrate and methane production tended to show lower abundance in MASLD, particularly in cirrhosis, while TMA- and SCA-producing genes were frequently enriched. These functional shifts were accompanied by the depletion of Agathobacter rectalis. Many of the altered genes were highly accessory and encoded on plasmids, suggesting genome-specific functional divergence driven by horizontal gene transfer.
CONCLUSION: MASLD is characterized by a shift toward alcohol- and TMA-producing metabolism, alongside reduced butyrate and methane production -changes driven by accessory and plasmid-borne genes. Gene-centric and mobile genetic element-aware profiling reveals mechanistic microbial contributions to MASLD that remain undetected by taxonomy-based approaches, offering new targets for diagnosis and intervention.
SUPPLEMENTARY INFORMATION: The online version contains supplementary material available at 10.1186/s12866-025-04549-5.
Additional Links: PMID-41413769
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41413769,
year = {2025},
author = {Medina-Méndez, JM and Iruzubieta, P and Fernández-López, R and Crespo, J and de la Cruz, F},
title = {Bacterial metabolic signatures in MASLD predicted through gene-centric studies in stool metagenomes.},
journal = {BMC microbiology},
volume = {26},
number = {1},
pages = {70},
pmid = {41413769},
issn = {1471-2180},
support = {PI22/01853//Spanish Carlos III Health Institute (ISCIII)/ ; PID2020-1179236B-100//Spanish MINECO/ ; },
abstract = {BACKGROUND: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a multifactorial condition in which the gut microbiome (GM) plays a central role. However, taxonomic associations derived from 16S ribosomal RNA (rRNA) gene studies have yielded inconsistent results, likely due to limited resolution and functional redundancy across taxa. We aimed to identify robust, functionally relevant microbial markers of MASLD using metagenomics and gene-centric profiling.
METHODS: We analyzed 554 fecal metagenomes from three independent cohorts. Sequencing reads were quality-controlled and taxonomically profiled with multi-marker gene resolution. We quantified the abundance of over 50 target gene families involved in butyrate, methane, trimethylamine (TMA) and short-chain alcohol (SCAs, i.e., ethanol and propanol) metabolism. Their presence was also determined across complete GM genomes and plasmids.
RESULTS: Genes involved in butyrate and methane production tended to show lower abundance in MASLD, particularly in cirrhosis, while TMA- and SCA-producing genes were frequently enriched. These functional shifts were accompanied by the depletion of Agathobacter rectalis. Many of the altered genes were highly accessory and encoded on plasmids, suggesting genome-specific functional divergence driven by horizontal gene transfer.
CONCLUSION: MASLD is characterized by a shift toward alcohol- and TMA-producing metabolism, alongside reduced butyrate and methane production -changes driven by accessory and plasmid-borne genes. Gene-centric and mobile genetic element-aware profiling reveals mechanistic microbial contributions to MASLD that remain undetected by taxonomy-based approaches, offering new targets for diagnosis and intervention.
SUPPLEMENTARY INFORMATION: The online version contains supplementary material available at 10.1186/s12866-025-04549-5.},
}
RevDate: 2026-01-29
Ecosystem-specific composition and drivers of plastisphere resistome in freshwater and marine environments.
Environmental research pii:S0013-9351(26)00186-6 [Epub ahead of print].
Microplastics in aquatic environments facilitate the formation of specific plastisphere microbiomes and serve as potential hotspots for antibiotic resistance genes (ARGs) propagation. However, the systematic comparisons of ARG profiles on microplastics from different aquatic ecosystems remain limited, particularly the prevalent ARGs and their bacterial hosts. This study performed a comparative meta-analysis of existing metagenomic datasets to investigate the resistome between freshwater and seawater microplastics (FMP and SMP) and their driving factors. Our results revealed that the ARG profiles on both FMP and SMP were significantly distinct from their surrounding waterbody. Moreover, FMP exhibited a higher diversity and abundance of ARGs rather than SMP. Ten core ARGs were shared on FMP and SMP, while 23 core ARGs were exclusively detected on FMP. The bacterial community on microplastics exhibited an ecosystem-specific composition, and was identified as the primary determinant shaping the ARG profiles. Notably, more complex bacteria-ARG co-occurrence pattern was identified on FMP, involving a broader spectrum of core genera and potential pathogenic hosts (e.g., Mycobacterium, Streptomyces). Furthermore, a significant and specific correlation between mobile genetic elements and ARGs was identified on FMP but not SMP, suggesting a markedly elevated horizontal gene transfer potential, with mechanistic support from the concurrent enrichment of oxidative stress and SOS response genes on FMP. These findings provide a comprehensive characterization of ARGs on aquatic microplastics, and especially highlight the role of FMP on the ARG dissemination.
Additional Links: PMID-41611051
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41611051,
year = {2026},
author = {Shi, J and Sun, C and Su, Y and Wu, Y and Zhan, M and Ji, C and Wang, R and Lv, B},
title = {Ecosystem-specific composition and drivers of plastisphere resistome in freshwater and marine environments.},
journal = {Environmental research},
volume = {},
number = {},
pages = {123858},
doi = {10.1016/j.envres.2026.123858},
pmid = {41611051},
issn = {1096-0953},
abstract = {Microplastics in aquatic environments facilitate the formation of specific plastisphere microbiomes and serve as potential hotspots for antibiotic resistance genes (ARGs) propagation. However, the systematic comparisons of ARG profiles on microplastics from different aquatic ecosystems remain limited, particularly the prevalent ARGs and their bacterial hosts. This study performed a comparative meta-analysis of existing metagenomic datasets to investigate the resistome between freshwater and seawater microplastics (FMP and SMP) and their driving factors. Our results revealed that the ARG profiles on both FMP and SMP were significantly distinct from their surrounding waterbody. Moreover, FMP exhibited a higher diversity and abundance of ARGs rather than SMP. Ten core ARGs were shared on FMP and SMP, while 23 core ARGs were exclusively detected on FMP. The bacterial community on microplastics exhibited an ecosystem-specific composition, and was identified as the primary determinant shaping the ARG profiles. Notably, more complex bacteria-ARG co-occurrence pattern was identified on FMP, involving a broader spectrum of core genera and potential pathogenic hosts (e.g., Mycobacterium, Streptomyces). Furthermore, a significant and specific correlation between mobile genetic elements and ARGs was identified on FMP but not SMP, suggesting a markedly elevated horizontal gene transfer potential, with mechanistic support from the concurrent enrichment of oxidative stress and SOS response genes on FMP. These findings provide a comprehensive characterization of ARGs on aquatic microplastics, and especially highlight the role of FMP on the ARG dissemination.},
}
RevDate: 2026-01-29
Isolation and whole-genome sequencing of antibiotic-resistant bacteria revealed the reservoir for indigenous antibiotic resistance genes in the deepest ocean sediment of the Challenger Deep.
Marine pollution bulletin, 226:119206 pii:S0025-326X(25)01682-0 [Epub ahead of print].
Understanding the occurrence of antibiotic-resistant bacteria (ARB) and associated antibiotic resistance genes (ARGs) in remote marine environments is crucial for accessing treats of ARG pollution on a border ecological scale. While most studies focused on anthropogenically disturbed settings, the Challenger Deep, as the deepest ocean habitat, offers a unique opportunity to investigate minimally disturbed resistomes. We revived 123 bacterial isolates from the Challenger Deep sediment, assessed their antibiotic susceptibility, and identified their taxonomy via 16S rRNA gene sequencing. Among them, 96 strains (78.0%) were resistant to at least one antibiotic, with high prevalence observed in Halomonas, Idiomarina, Flagellimonas, and Microbacterium. Resistance was most common to ampicillin (73.2%), followed by sulfadiazine (30.1%) and nalidixic acid (4.9%). Untargeted metabolomics identified 359 metabolites in the sediment sample, including 6-aminopenicillanic acid, suggesting local microbial antibiotic production and selective pressure of resistance. Anthropogenic contaminants like nalidixic acid were also detected. Whole-genome sequencing of eight representative ARB strains revealed 77 copies of 26 ARG subtypes, predominantly associated with multidrug resistance and efflux pump mechanisms. Notably, no mobile genetic elements were linked to ARGs, indicating limited horizontal gene transfer. Phylogenetic analyses showed host species specificity of ARGs, independent of geography or environmental context, supporting vertical inheritance from ancestral lineages. This study offers the first culture-based evidence of ARB and ARGs in the Challenger Deep, suggesting that resistance may represent an adaptive trait to extreme conditions and underscoring its ancient, intrinsic origin. Our findings provide critical implications for understanding the revolution and dissemination of resistance in deep-sea environments.
Additional Links: PMID-41610533
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41610533,
year = {2026},
author = {Ding, W and Wang, Y and Ma, Y and Chen, P and Yang, J and Song, Z and Wang, Y and Zhang, W and Li, X and Huang, Y and Nan, P},
title = {Isolation and whole-genome sequencing of antibiotic-resistant bacteria revealed the reservoir for indigenous antibiotic resistance genes in the deepest ocean sediment of the Challenger Deep.},
journal = {Marine pollution bulletin},
volume = {226},
number = {},
pages = {119206},
doi = {10.1016/j.marpolbul.2025.119206},
pmid = {41610533},
issn = {1879-3363},
abstract = {Understanding the occurrence of antibiotic-resistant bacteria (ARB) and associated antibiotic resistance genes (ARGs) in remote marine environments is crucial for accessing treats of ARG pollution on a border ecological scale. While most studies focused on anthropogenically disturbed settings, the Challenger Deep, as the deepest ocean habitat, offers a unique opportunity to investigate minimally disturbed resistomes. We revived 123 bacterial isolates from the Challenger Deep sediment, assessed their antibiotic susceptibility, and identified their taxonomy via 16S rRNA gene sequencing. Among them, 96 strains (78.0%) were resistant to at least one antibiotic, with high prevalence observed in Halomonas, Idiomarina, Flagellimonas, and Microbacterium. Resistance was most common to ampicillin (73.2%), followed by sulfadiazine (30.1%) and nalidixic acid (4.9%). Untargeted metabolomics identified 359 metabolites in the sediment sample, including 6-aminopenicillanic acid, suggesting local microbial antibiotic production and selective pressure of resistance. Anthropogenic contaminants like nalidixic acid were also detected. Whole-genome sequencing of eight representative ARB strains revealed 77 copies of 26 ARG subtypes, predominantly associated with multidrug resistance and efflux pump mechanisms. Notably, no mobile genetic elements were linked to ARGs, indicating limited horizontal gene transfer. Phylogenetic analyses showed host species specificity of ARGs, independent of geography or environmental context, supporting vertical inheritance from ancestral lineages. This study offers the first culture-based evidence of ARB and ARGs in the Challenger Deep, suggesting that resistance may represent an adaptive trait to extreme conditions and underscoring its ancient, intrinsic origin. Our findings provide critical implications for understanding the revolution and dissemination of resistance in deep-sea environments.},
}
RevDate: 2026-01-29
Genomic characterization of colistin- and carbapenem-resistant Pseudomonas aeruginosa ST1560 from Guanabara Bay, Brazil.
Journal of applied microbiology pii:8444579 [Epub ahead of print].
AIMS: This study aimed to characterize a colistin- and carbapenem-resistant Pseudomonas aeruginosa ST1560 strain isolated from Guanabara Bay, Brazil, and to investigate the molecular mechanisms underlying its resistance phenotype.
METHODS AND RESULTS: Six surface water samples from Guanabara Bay were collected, yielding 71 P. aeruginosa subjected to antimicrobial susceptibility testing. Three isolates exhibited elevated minimal inhibitory concentrations (MICs) to colistin (≥512, 64, and 8 mg/L) in the absence of mcr genes (1-10). Among these, only strain CCVSU 5861 demonstrated carbapenemase confirmed by Blue Carba test. This strain was selected for whole-genome sequencing (Illumina). Genomic analysis identified the presence of blaKPC-2 and blaOXA-395, along with additional resistance determinants associated with aminoglycosides and fosfomycin. Genes involved in lipopolysaccharide modification, (arnA, arnT, and basS) were also detected, likely contributing to colistin resistance. The blaKPC-2 gene was located adjacent to the mobile genetic element ISKpn6, suggesting potential horizontal gene transfer.
CONCLUSIONS: The P. aeruginosa ST1560 displays a complex multidrug resistance profile, including resistance to both colistin and carbapenems. This phenotype appears to be mediated by a combination of acquired resistance genes and chromosomal mechanisms. The localization of blaKPC-2 within a mobile genetic element underscores the risk of dissemination in aquatic environments.
Additional Links: PMID-41609416
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41609416,
year = {2026},
author = {Assunção, VC and Magaldi, M and Lopes-Carvalho, M and Santos, HSO and Gonçalves-Britto, AS and Vianna, TCC and de Souza, HDF and Montenegro, K and Paranhos, R and Cardoso, AM and Bianco, K and Clementino, MM},
title = {Genomic characterization of colistin- and carbapenem-resistant Pseudomonas aeruginosa ST1560 from Guanabara Bay, Brazil.},
journal = {Journal of applied microbiology},
volume = {},
number = {},
pages = {},
doi = {10.1093/jambio/lxag035},
pmid = {41609416},
issn = {1365-2672},
abstract = {AIMS: This study aimed to characterize a colistin- and carbapenem-resistant Pseudomonas aeruginosa ST1560 strain isolated from Guanabara Bay, Brazil, and to investigate the molecular mechanisms underlying its resistance phenotype.
METHODS AND RESULTS: Six surface water samples from Guanabara Bay were collected, yielding 71 P. aeruginosa subjected to antimicrobial susceptibility testing. Three isolates exhibited elevated minimal inhibitory concentrations (MICs) to colistin (≥512, 64, and 8 mg/L) in the absence of mcr genes (1-10). Among these, only strain CCVSU 5861 demonstrated carbapenemase confirmed by Blue Carba test. This strain was selected for whole-genome sequencing (Illumina). Genomic analysis identified the presence of blaKPC-2 and blaOXA-395, along with additional resistance determinants associated with aminoglycosides and fosfomycin. Genes involved in lipopolysaccharide modification, (arnA, arnT, and basS) were also detected, likely contributing to colistin resistance. The blaKPC-2 gene was located adjacent to the mobile genetic element ISKpn6, suggesting potential horizontal gene transfer.
CONCLUSIONS: The P. aeruginosa ST1560 displays a complex multidrug resistance profile, including resistance to both colistin and carbapenems. This phenotype appears to be mediated by a combination of acquired resistance genes and chromosomal mechanisms. The localization of blaKPC-2 within a mobile genetic element underscores the risk of dissemination in aquatic environments.},
}
RevDate: 2026-01-29
CmpDate: 2026-01-29
Decoding resistome profiles and horizontal transfer of antibiotic resistance genes across the pork production chain under One Health sectors.
Food research international (Ottawa, Ont.), 221(Pt 1):117259.
The emergence of antimicrobial resistance has become a global threat to public health. Intensive antibiotic use in swine farming has accelerated the proliferation of antibiotic resistance genes (ARGs) in animal-derived foods, making the production chain a potential ARG transmission route to humans. However, shared resistome profiles and horizontal gene transfer (HGT) mechanisms along this chain remain unclear. Here, we systematically investigated the resistome profile, ARGs' host, and potential HGT of ARGs across interconnected swine farm, slaughterhouse, and retail market by metagenomic assembly and binning. From 42 metagenomes, 1354 ARG subtypes were identified, with 303 shared across all interfaces. Both microbiome and mobile genetic elements (MGEs) contributed to the variation in ARG profiles. Pseudomonadota were the dominant drivers that shape the resistome through plasmid-mediated HGT. Among the 133 reconstructed ARG-carrying genomes (ACGs), 38 of them carried multiple ARGs, indicating the potential mobility of ARGs. Notably, 3 ACGs taxonomically assigned to Pseudomonas_E alcaligenes, Serratia_J grimesii, and Escherichia coli carrying 9, 13, and 41 ARGs, respectively. Furthermore, MetaCHIP analysis uncovered 445 potential HGT events, and ARGs including CpxR, macB, fusA, and vanR were annotated as potentially transferred subtypes. This study decodes the resistome profiles and tracks horizontal ARG transfer at the community level across the entire pork supply chain - from swine farms to retail outlets. To our knowledge, few studies have explored ARG transmission subtypes and directional flows among humans, pigs, and environmental compartments in the pork production chain using metagenomic approaches. These findings highlight the important role of the pork production chain as a critical transmission vector for ARGs under One Health framework.
Additional Links: PMID-41606855
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41606855,
year = {2025},
author = {Yang, J and He, Y and Huang, J and Li, M and Wu, X and Pei, X and Yang, X},
title = {Decoding resistome profiles and horizontal transfer of antibiotic resistance genes across the pork production chain under One Health sectors.},
journal = {Food research international (Ottawa, Ont.)},
volume = {221},
number = {Pt 1},
pages = {117259},
doi = {10.1016/j.foodres.2025.117259},
pmid = {41606855},
issn = {1873-7145},
mesh = {*Gene Transfer, Horizontal ; Animals ; Swine ; Anti-Bacterial Agents/pharmacology ; *Drug Resistance, Microbial/genetics ; *One Health ; *Drug Resistance, Bacterial/genetics ; *Pork Meat/microbiology ; Food Microbiology ; Abattoirs ; Metagenome ; Microbiota/genetics ; Metagenomics ; Bacteria/genetics ; },
abstract = {The emergence of antimicrobial resistance has become a global threat to public health. Intensive antibiotic use in swine farming has accelerated the proliferation of antibiotic resistance genes (ARGs) in animal-derived foods, making the production chain a potential ARG transmission route to humans. However, shared resistome profiles and horizontal gene transfer (HGT) mechanisms along this chain remain unclear. Here, we systematically investigated the resistome profile, ARGs' host, and potential HGT of ARGs across interconnected swine farm, slaughterhouse, and retail market by metagenomic assembly and binning. From 42 metagenomes, 1354 ARG subtypes were identified, with 303 shared across all interfaces. Both microbiome and mobile genetic elements (MGEs) contributed to the variation in ARG profiles. Pseudomonadota were the dominant drivers that shape the resistome through plasmid-mediated HGT. Among the 133 reconstructed ARG-carrying genomes (ACGs), 38 of them carried multiple ARGs, indicating the potential mobility of ARGs. Notably, 3 ACGs taxonomically assigned to Pseudomonas_E alcaligenes, Serratia_J grimesii, and Escherichia coli carrying 9, 13, and 41 ARGs, respectively. Furthermore, MetaCHIP analysis uncovered 445 potential HGT events, and ARGs including CpxR, macB, fusA, and vanR were annotated as potentially transferred subtypes. This study decodes the resistome profiles and tracks horizontal ARG transfer at the community level across the entire pork supply chain - from swine farms to retail outlets. To our knowledge, few studies have explored ARG transmission subtypes and directional flows among humans, pigs, and environmental compartments in the pork production chain using metagenomic approaches. These findings highlight the important role of the pork production chain as a critical transmission vector for ARGs under One Health framework.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Gene Transfer, Horizontal
Animals
Swine
Anti-Bacterial Agents/pharmacology
*Drug Resistance, Microbial/genetics
*One Health
*Drug Resistance, Bacterial/genetics
*Pork Meat/microbiology
Food Microbiology
Abattoirs
Metagenome
Microbiota/genetics
Metagenomics
Bacteria/genetics
RevDate: 2026-01-29
Cell density and single-cell heterogeneity reveal distinct competence induction dynamics in the high-GC Gram-positive Micrococcus luteus.
BMC microbiology, 26(1):63.
BACKGROUND: Competence for natural transformation enables bacteria to acquire extracellular DNA and incorporate it into their genome, driving genetic diversification, DNA repair, and adaptation. While the regulatory mechanisms of competence development are well characterized in model organisms such as Bacillus subtilis and Streptococcus pneumoniae, little is known about how this process is controlled in Actinomycetota. Here, we investigate competence development in Micrococcus luteus, a high-GC Gram-positive species historically recognized for natural transformation.
RESULTS: Using transformation frequency assays, transcriptional reporters, single-cell flow cytometry, and fluorescence microscopy, we show that in this actinobacterial model competence is consistent with a probabilistic regulatory strategy that integrates cell density, nutrient-limitation-responsive signals, and physiological state. Peak transformation occurs during exponential growth in minimal medium at moderate inoculation densities, whereas both low and high starting densities suppress competence. Although transcription of the late competence genes comEA/EC is induced under competence-promoting conditions, this activation does not always correlate with transformability, indicating additional post-transcriptional or physiological regulation. Single-cell analyses revealed that promoter activity develops gradually and heterogeneously across the population, lacking the bistability or strong population-level coordination observed in other well-studied Gram-positive model systems.
CONCLUSIONS: These data characterize competence induction dynamics in M. luteus and expand our understanding of the diversity of competence regulation across bacteria. While these observations constrain plausible regulatory models—supporting density- and nutrient-sensitive, probabilistic induction with heterogeneous single-cell activation—the upstream signal(s) or regulatory cascade controlling competence in M. luteus remain to be identified. Together, the results suggest that high-GC Gram-positive Actinomycetota may employ distinct, potentially bet-hedging-like strategies to balance growth, stress responses, and horizontal gene transfer.
SUPPLEMENTARY INFORMATION: The online version contains supplementary material available at 10.1186/s12866-026-04757-7.
Additional Links: PMID-41572162
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41572162,
year = {2026},
author = {Lichev, A and Angelov, A and Liebl, W},
title = {Cell density and single-cell heterogeneity reveal distinct competence induction dynamics in the high-GC Gram-positive Micrococcus luteus.},
journal = {BMC microbiology},
volume = {26},
number = {1},
pages = {63},
pmid = {41572162},
issn = {1471-2180},
abstract = {BACKGROUND: Competence for natural transformation enables bacteria to acquire extracellular DNA and incorporate it into their genome, driving genetic diversification, DNA repair, and adaptation. While the regulatory mechanisms of competence development are well characterized in model organisms such as Bacillus subtilis and Streptococcus pneumoniae, little is known about how this process is controlled in Actinomycetota. Here, we investigate competence development in Micrococcus luteus, a high-GC Gram-positive species historically recognized for natural transformation.
RESULTS: Using transformation frequency assays, transcriptional reporters, single-cell flow cytometry, and fluorescence microscopy, we show that in this actinobacterial model competence is consistent with a probabilistic regulatory strategy that integrates cell density, nutrient-limitation-responsive signals, and physiological state. Peak transformation occurs during exponential growth in minimal medium at moderate inoculation densities, whereas both low and high starting densities suppress competence. Although transcription of the late competence genes comEA/EC is induced under competence-promoting conditions, this activation does not always correlate with transformability, indicating additional post-transcriptional or physiological regulation. Single-cell analyses revealed that promoter activity develops gradually and heterogeneously across the population, lacking the bistability or strong population-level coordination observed in other well-studied Gram-positive model systems.
CONCLUSIONS: These data characterize competence induction dynamics in M. luteus and expand our understanding of the diversity of competence regulation across bacteria. While these observations constrain plausible regulatory models—supporting density- and nutrient-sensitive, probabilistic induction with heterogeneous single-cell activation—the upstream signal(s) or regulatory cascade controlling competence in M. luteus remain to be identified. Together, the results suggest that high-GC Gram-positive Actinomycetota may employ distinct, potentially bet-hedging-like strategies to balance growth, stress responses, and horizontal gene transfer.
SUPPLEMENTARY INFORMATION: The online version contains supplementary material available at 10.1186/s12866-026-04757-7.},
}
RevDate: 2026-01-29
CmpDate: 2026-01-29
Roles of micro/nanoplastics in the spread of antimicrobial resistance through conjugative gene transfer.
Nature communications, 17(1):1118.
The role of micro/nanoplastics (M/NPs) in the dissemination of antimicrobial resistance (AMR) remains insufficiently understood. Here, we examine how polystyrene (PS) M/NPs of varying sizes and concentrations affect AMR gene (ARG) transfer in model systems with gram-negative (Escherichia coli) and gram-positive (Enterococcus faecalis) donors. In these systems, the ARG transfer frequency is higher for intrageneric pairs than for intergeneric pairs. The 20- and 120-nm-sized PS broadly facilitate conjugation, whereas the 1-μm-sized PS selectively promotes ARG transfer to E. coli recipients, in addition to altering the expression of conjugation- and pili-associated genes. Notably, an environmentally relevant (0.1 mg/L) concentration of PS M/NPs facilitates AMR transfer in the tested systems, which correlates with increased reactive oxygen species levels, ATP levels, and cell membrane permeability in both donors and recipients. Collectively, our findings underscore the role of M/NPs in facilitating AMR spread in specific bacterial systems, providing valuable insights for understanding their potential ecological risk in water environments.
Additional Links: PMID-41444368
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41444368,
year = {2025},
author = {Kang, Y and Gao, SH and Pan, Y and Gao, R and Li, T and Fan, L and Su, Y and Zhang, W and Yu, Z and Liang, B and Su, JQ and Luo, Y and Wang, Y and Guo, J and Wang, A},
title = {Roles of micro/nanoplastics in the spread of antimicrobial resistance through conjugative gene transfer.},
journal = {Nature communications},
volume = {17},
number = {1},
pages = {1118},
pmid = {41444368},
issn = {2041-1723},
support = {52321005, 52070060, 52230004 and 52293441//National Natural Science Foundation of China (National Science Foundation of China)/ ; 2024A1515010085//Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)/ ; GXWD20231127195344001 and JCYJ20241202123735045//Shenzhen Science and Technology Innovation Commission/ ; },
mesh = {*Escherichia coli/genetics/drug effects ; Polystyrenes/chemistry/pharmacology ; *Conjugation, Genetic/drug effects ; *Drug Resistance, Bacterial/genetics ; *Enterococcus faecalis/genetics/drug effects ; *Gene Transfer, Horizontal ; Anti-Bacterial Agents/pharmacology ; *Microplastics/chemistry ; Reactive Oxygen Species/metabolism ; *Nanoparticles/chemistry ; Adenosine Triphosphate/metabolism ; },
abstract = {The role of micro/nanoplastics (M/NPs) in the dissemination of antimicrobial resistance (AMR) remains insufficiently understood. Here, we examine how polystyrene (PS) M/NPs of varying sizes and concentrations affect AMR gene (ARG) transfer in model systems with gram-negative (Escherichia coli) and gram-positive (Enterococcus faecalis) donors. In these systems, the ARG transfer frequency is higher for intrageneric pairs than for intergeneric pairs. The 20- and 120-nm-sized PS broadly facilitate conjugation, whereas the 1-μm-sized PS selectively promotes ARG transfer to E. coli recipients, in addition to altering the expression of conjugation- and pili-associated genes. Notably, an environmentally relevant (0.1 mg/L) concentration of PS M/NPs facilitates AMR transfer in the tested systems, which correlates with increased reactive oxygen species levels, ATP levels, and cell membrane permeability in both donors and recipients. Collectively, our findings underscore the role of M/NPs in facilitating AMR spread in specific bacterial systems, providing valuable insights for understanding their potential ecological risk in water environments.},
}
MeSH Terms:
show MeSH Terms
hide MeSH Terms
*Escherichia coli/genetics/drug effects
Polystyrenes/chemistry/pharmacology
*Conjugation, Genetic/drug effects
*Drug Resistance, Bacterial/genetics
*Enterococcus faecalis/genetics/drug effects
*Gene Transfer, Horizontal
Anti-Bacterial Agents/pharmacology
*Microplastics/chemistry
Reactive Oxygen Species/metabolism
*Nanoparticles/chemistry
Adenosine Triphosphate/metabolism
RevDate: 2026-01-28
A bacterial gene acquired by parasitoid wasps contributes to venom secretion against host defence.
The EMBO journal [Epub ahead of print].
Horizontal gene transfer (HGT) is an important source of gene innovation in prokaryotic and eukaryotic organisms. Several genes acquired by hosts of parasitoid wasps via HGT have been reported to protect hosts from parasitoid wasps. In contrast, little is known about whether HGT-acquired genes in parasitoid wasps are involved in attacking their hosts. Here, we report a prokaryote-type CDP-diacylglycerol synthase (PTCDS) gene that was horizontally transferred into the last common ancestor of two parasitoid wasps, Leptopilina heterotoma and L. syphax, from the bacterial family Rickettsiaceae. We experimentally demonstrated that PTCDS is linked to ensure the appropriate storage amount of venom in the venom reservoir of parasitoid wasps. PTCDS knockdown downregulated the expression of certain vesicle-mediated transport genes, thereby reducing the secretion of venom into venom reservoir without altering its composition. This resulted in a significant increase in the proportion of encapsulated wasp eggs in parasitized hosts, ultimately leading to host immune-mediated killing. We conclude that parasitoid wasps use the foreign gene PTCDS to influence venom amounts against host defence, providing new insight into the arms race between parasitoid wasps and hosts.
Additional Links: PMID-41606198
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41606198,
year = {2026},
author = {Liu, Z and Tao, M and Xu, Z and Zhang, J and Li, Y and Dong, Z and Zhang, Q and Pang, L and Sheng, Y and Lu, Y and Feng, T and Shi, W and Yu, L and Rokas, A and Chen, J and Shen, XX and Huang, J},
title = {A bacterial gene acquired by parasitoid wasps contributes to venom secretion against host defence.},
journal = {The EMBO journal},
volume = {},
number = {},
pages = {},
pmid = {41606198},
issn = {1460-2075},
support = {32325044//MOST | National Natural Science Foundation of China (NSFC)/ ; 32172467//MOST | National Natural Science Foundation of China (NSFC)/ ; 32071665//MOST | National Natural Science Foundation of China (NSFC)/ ; 32202375//MOST | National Natural Science Foundation of China (NSFC)/ ; LZ23C140003//Zhejiang Provincial Natural Science Foundation of China/ ; 2022YFD1401600//MOST | National Key Research and Development Program of China (NKPs)/ ; LR23C140001//National Science Foundation for Distinguished Young Scholars of Zhejiang Province/ ; DEB-2110404//National Science Foundation (NSF)/ ; R01 AI153356/AI/NIAID NIH HHS/United States ; },
abstract = {Horizontal gene transfer (HGT) is an important source of gene innovation in prokaryotic and eukaryotic organisms. Several genes acquired by hosts of parasitoid wasps via HGT have been reported to protect hosts from parasitoid wasps. In contrast, little is known about whether HGT-acquired genes in parasitoid wasps are involved in attacking their hosts. Here, we report a prokaryote-type CDP-diacylglycerol synthase (PTCDS) gene that was horizontally transferred into the last common ancestor of two parasitoid wasps, Leptopilina heterotoma and L. syphax, from the bacterial family Rickettsiaceae. We experimentally demonstrated that PTCDS is linked to ensure the appropriate storage amount of venom in the venom reservoir of parasitoid wasps. PTCDS knockdown downregulated the expression of certain vesicle-mediated transport genes, thereby reducing the secretion of venom into venom reservoir without altering its composition. This resulted in a significant increase in the proportion of encapsulated wasp eggs in parasitized hosts, ultimately leading to host immune-mediated killing. We conclude that parasitoid wasps use the foreign gene PTCDS to influence venom amounts against host defence, providing new insight into the arms race between parasitoid wasps and hosts.},
}
RevDate: 2026-01-28
Comparative genomics reveals two major lineages of Bifidobacterium adolescentis in the human gut, driven by divergent adaptation in China and the United States.
Journal of advanced research pii:S2090-1232(26)00098-6 [Epub ahead of print].
INTRODUCTION: Bifidobacterium adolescentis is a key beneficial member of the human gut microbiota, but its genomic diversity and evolutionary drivers across human populations remain poorly characterized.
OBJECTIVES: Understanding genomic functional heterogeneity and evolutionary patterns in human gut-derived B. adolescentis.
METHODS: We performed a comparative genomic analysis of 395B. adolescentis, mainly from China (n = 169) and the U.S. (n = 146), with smaller sets from Australia, Italy, and the United Kingdom, to investigate functional heterogeneity and evolutionary mechanisms. Our analysis integrated core and pan-genome architecture, phylogenomics, single nucleotide polymorphism (SNP)-based population structure, carbohydrate-active enzyme profiles, CRISPR-Cas systems, antibiotic resistance genes, and recombination dynamics.
RESULTS: The pan-genome was open and highly plastic. Phylogenetic reconstruction identified two major clades with strong geographic stratification: Chinese isolates predominantly clustered in Clade B, while U.S. isolates grouped in Clade A. Functional annotation showed regional specialization in carbohydrate-active enzymes, with Chinese isolates enriched in glycosyltransferase families and U.S. isolates in carbohydrate-binding module and carboxylesterase families, likely reflecting dietary adaptations. Genomic islands were hotspots for horizontal gene transfer, harboring region-specific carbohydrate-active enzymes and antibiotic resistance genes such as tet(W/32/O) and ermX, which were frequently located in Chinese isolates. Recombination was found to be the primary driver of genetic diversity, with recombination-to-mutation ratios approaching and exceeding 3.0 in Chinese and U.S. isolates. Linkage disequilibrium decay further supported higher recombination rates in these populations.
CONCLUSION: B. adolescentis has diverged into two major genomic lineages, primarily associated with isolates from China and the U.S. This divergence reflects adaptation to distinct host-associated ecological factors, such as diet, antibiotic exposure, and lifestyle, and is predominantly driven by extensive homologous recombination rather than point mutations. These findings highlight how regional selective pressures shape the genomic and functional landscape of this key gut symbiont.
Additional Links: PMID-41605290
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41605290,
year = {2026},
author = {Shi, L and Zhang, M and Zheng, R and Kwok, LY and Zhang, W},
title = {Comparative genomics reveals two major lineages of Bifidobacterium adolescentis in the human gut, driven by divergent adaptation in China and the United States.},
journal = {Journal of advanced research},
volume = {},
number = {},
pages = {},
doi = {10.1016/j.jare.2026.01.071},
pmid = {41605290},
issn = {2090-1224},
abstract = {INTRODUCTION: Bifidobacterium adolescentis is a key beneficial member of the human gut microbiota, but its genomic diversity and evolutionary drivers across human populations remain poorly characterized.
OBJECTIVES: Understanding genomic functional heterogeneity and evolutionary patterns in human gut-derived B. adolescentis.
METHODS: We performed a comparative genomic analysis of 395B. adolescentis, mainly from China (n = 169) and the U.S. (n = 146), with smaller sets from Australia, Italy, and the United Kingdom, to investigate functional heterogeneity and evolutionary mechanisms. Our analysis integrated core and pan-genome architecture, phylogenomics, single nucleotide polymorphism (SNP)-based population structure, carbohydrate-active enzyme profiles, CRISPR-Cas systems, antibiotic resistance genes, and recombination dynamics.
RESULTS: The pan-genome was open and highly plastic. Phylogenetic reconstruction identified two major clades with strong geographic stratification: Chinese isolates predominantly clustered in Clade B, while U.S. isolates grouped in Clade A. Functional annotation showed regional specialization in carbohydrate-active enzymes, with Chinese isolates enriched in glycosyltransferase families and U.S. isolates in carbohydrate-binding module and carboxylesterase families, likely reflecting dietary adaptations. Genomic islands were hotspots for horizontal gene transfer, harboring region-specific carbohydrate-active enzymes and antibiotic resistance genes such as tet(W/32/O) and ermX, which were frequently located in Chinese isolates. Recombination was found to be the primary driver of genetic diversity, with recombination-to-mutation ratios approaching and exceeding 3.0 in Chinese and U.S. isolates. Linkage disequilibrium decay further supported higher recombination rates in these populations.
CONCLUSION: B. adolescentis has diverged into two major genomic lineages, primarily associated with isolates from China and the U.S. This divergence reflects adaptation to distinct host-associated ecological factors, such as diet, antibiotic exposure, and lifestyle, and is predominantly driven by extensive homologous recombination rather than point mutations. These findings highlight how regional selective pressures shape the genomic and functional landscape of this key gut symbiont.},
}
RevDate: 2026-01-28
CmpDate: 2026-01-28
Nanocarrier-mediated CRISPR-Cas delivery: a novel approach against antibiotic-resistant superbugs.
Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society, 34(1):5.
Antibiotic resistance (ABR) is a leading cause of death and a major public health threat globally. Without appropriate interventions, annual ABR-associated deaths have been projected to reach 10 million by 2050 worldwide. Hence, it is critical to develop novel therapeutic interventions that would be able to tackle ABR by targeting mainly the pathogenic microbes, while lessening harm to beneficial microbes. There is an increasing research interest in CRISPR-Cas (CC) systems owing to their potential in controlling and preventing horizontal gene transfer and spread of antibiotic resistance. In addition, CC systems offer several advantages, including high efficiency, rapid turnaround time, low cost, and easy design, which allow these systems to effectively and precisely target antibiotic-resistant bacteria. CRISPR-based gene therapy offers numerous benefits; however, the major limitation in clinical translation is the safe and effective delivery of CRISPR components to target organs or cells, thus hindering its potential in therapeutic interventions. Nanocarriers (NCs) can help the CC systems to overcome their off-target effects by precisely delivering the systems to the target cells. NCs can also be engineered for target site release, payload protection, and high specificity, which can further ensure delivery of the components of CC in the target cells or regions without harming surrounding tissues. This review summarizes the principles and mechanisms of CC systems, highlights their applications against antibiotic-resistant bacteria, and discusses emerging nanocarrier-based delivery strategies that may enhance the clinical utility of CRISPR-Cas technologies in managing ABR.
Additional Links: PMID-41604096
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41604096,
year = {2026},
author = {Almufarriji, FM},
title = {Nanocarrier-mediated CRISPR-Cas delivery: a novel approach against antibiotic-resistant superbugs.},
journal = {Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society},
volume = {34},
number = {1},
pages = {5},
pmid = {41604096},
issn = {1319-0164},
abstract = {Antibiotic resistance (ABR) is a leading cause of death and a major public health threat globally. Without appropriate interventions, annual ABR-associated deaths have been projected to reach 10 million by 2050 worldwide. Hence, it is critical to develop novel therapeutic interventions that would be able to tackle ABR by targeting mainly the pathogenic microbes, while lessening harm to beneficial microbes. There is an increasing research interest in CRISPR-Cas (CC) systems owing to their potential in controlling and preventing horizontal gene transfer and spread of antibiotic resistance. In addition, CC systems offer several advantages, including high efficiency, rapid turnaround time, low cost, and easy design, which allow these systems to effectively and precisely target antibiotic-resistant bacteria. CRISPR-based gene therapy offers numerous benefits; however, the major limitation in clinical translation is the safe and effective delivery of CRISPR components to target organs or cells, thus hindering its potential in therapeutic interventions. Nanocarriers (NCs) can help the CC systems to overcome their off-target effects by precisely delivering the systems to the target cells. NCs can also be engineered for target site release, payload protection, and high specificity, which can further ensure delivery of the components of CC in the target cells or regions without harming surrounding tissues. This review summarizes the principles and mechanisms of CC systems, highlights their applications against antibiotic-resistant bacteria, and discusses emerging nanocarrier-based delivery strategies that may enhance the clinical utility of CRISPR-Cas technologies in managing ABR.},
}
RevDate: 2026-01-28
Genomic and proteomic analyses of the maize root isolate Rhodococcus erythropolis NI86/21 reveal extensive genome plasticity and parallel evolution of herbicide degradation.
Applied and environmental microbiology [Epub ahead of print].
Rhodococcus erythropolis NI86/21, isolated from maize rhizosphere in Hungary, possesses one of the largest genomes (8.046 Mb) within the species. The genome comprises a 6.83 Mb chromosome and 1.22 Mb of extrachromosomal elements, including three circular and two fragmented linear plasmids. Comparative analysis identified five horizontally acquired genomic islands (HGTi), totaling 0.64 Mb with mosaic-like architecture derived from plasmids, phages, and chromosomal segments of other Nocardiaceae. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomic analysis revealed a lower expression of genes located in HGT elements (53%) compared to core chromosomal genes (73%), indicating regulatory silencing of foreign DNA. Nevertheless, an inducible cytochrome P450 monooxygenase (CYP116) responsible for thiocarbamate and atrazine degradation is encoded on HGTi_V and actively expressed upon herbicide exposure. Strikingly, an identical CYP450 locus is present on a conjugative plasmid in Rhodococcus sp. TE1 isolated from thiocarbamate-treated soil in Canada, demonstrating independent acquisition of the same catabolic module from a high GC% content Rhodococcus, under similar selective pressure. Frequent recombination between chromosomal and mobile elements generates the observed mosaic-like HGT structures, which we found common for R. erythropolis strains. These results highlight extraordinary genomic plasticity and rapid adaptive evolution in Rhodococci, enabling efficient colonization of herbicide-contaminated agro-ecosystems.IMPORTANCERhodococcus erythropolis NI86/21 exemplifies how bacterial genomes evolve through horizontal gene transfer and mobile elements. Its unusually large, plastic genome contains extensive HGT islands and a high load of active transposons, which shape mosaic genomic architectures and hinder complete genome assembly. These horizontally acquired regions, although partially silenced, encode key adaptive functions such as an inducible CYP116 monooxygenase enabling thiocarbamate and atrazine degradation. Remarkably, an identical CYP116 module is present in Rhodococcus sp. TE1 from thiocarbamate-treated Canadian soil, demonstrating that similar environmental pressures can drive independent acquisition of the same biodegradation trait. Together, the dynamic transposon activity, mosaic HGT structure, and geographically convergent gene recruitment highlight the extraordinary genomic plasticity of R. erythropolis and underscore its rapid adaptive potential in agro-ecosystems, with implications for microbial evolution and bioremediation strategies.
Additional Links: PMID-41603638
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41603638,
year = {2026},
author = {Kosztik, J and Baka, E and Táncsics, A and Ábrahám, R and Szabó, G and Nagy, I and Orsini, M and Bata-Vidács, I and Szalontai, H and Kukolya, J and Nagy, I},
title = {Genomic and proteomic analyses of the maize root isolate Rhodococcus erythropolis NI86/21 reveal extensive genome plasticity and parallel evolution of herbicide degradation.},
journal = {Applied and environmental microbiology},
volume = {},
number = {},
pages = {e0240725},
doi = {10.1128/aem.02407-25},
pmid = {41603638},
issn = {1098-5336},
abstract = {Rhodococcus erythropolis NI86/21, isolated from maize rhizosphere in Hungary, possesses one of the largest genomes (8.046 Mb) within the species. The genome comprises a 6.83 Mb chromosome and 1.22 Mb of extrachromosomal elements, including three circular and two fragmented linear plasmids. Comparative analysis identified five horizontally acquired genomic islands (HGTi), totaling 0.64 Mb with mosaic-like architecture derived from plasmids, phages, and chromosomal segments of other Nocardiaceae. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomic analysis revealed a lower expression of genes located in HGT elements (53%) compared to core chromosomal genes (73%), indicating regulatory silencing of foreign DNA. Nevertheless, an inducible cytochrome P450 monooxygenase (CYP116) responsible for thiocarbamate and atrazine degradation is encoded on HGTi_V and actively expressed upon herbicide exposure. Strikingly, an identical CYP450 locus is present on a conjugative plasmid in Rhodococcus sp. TE1 isolated from thiocarbamate-treated soil in Canada, demonstrating independent acquisition of the same catabolic module from a high GC% content Rhodococcus, under similar selective pressure. Frequent recombination between chromosomal and mobile elements generates the observed mosaic-like HGT structures, which we found common for R. erythropolis strains. These results highlight extraordinary genomic plasticity and rapid adaptive evolution in Rhodococci, enabling efficient colonization of herbicide-contaminated agro-ecosystems.IMPORTANCERhodococcus erythropolis NI86/21 exemplifies how bacterial genomes evolve through horizontal gene transfer and mobile elements. Its unusually large, plastic genome contains extensive HGT islands and a high load of active transposons, which shape mosaic genomic architectures and hinder complete genome assembly. These horizontally acquired regions, although partially silenced, encode key adaptive functions such as an inducible CYP116 monooxygenase enabling thiocarbamate and atrazine degradation. Remarkably, an identical CYP116 module is present in Rhodococcus sp. TE1 from thiocarbamate-treated Canadian soil, demonstrating that similar environmental pressures can drive independent acquisition of the same biodegradation trait. Together, the dynamic transposon activity, mosaic HGT structure, and geographically convergent gene recruitment highlight the extraordinary genomic plasticity of R. erythropolis and underscore its rapid adaptive potential in agro-ecosystems, with implications for microbial evolution and bioremediation strategies.},
}
RevDate: 2026-01-28
Modeling and Functional Characterization of Reconstituted Efflux Pump Components from Heterologous Gram-Negative Bacteria.
ACS infectious diseases [Epub ahead of print].
Efflux pumps operating in bacteria continuously evolve and contribute significantly toward the rising global trends in antimicrobial resistance (AMR). Our earlier studies demonstrated that the expression of tripartite resistance nodulation division (RND) efflux pump containing the outer membrane protein (OMP), membrane fusion protein (MFP), and inner RND pump from different Gram-negative bacteria results in elevated minimum inhibitory concentrations (MICs) of different antibiotics. Interestingly, parts of this complex could be transferred either within the species or across genera. Despite limited sequence homology, we report the existence of significant structural and functional conservation between the distantly related MFP and RND proteins. Following the assembly of MFP components (AcrA, MexA, OqxA) and RND components (AcrB, MexB, OqxB) from E. coli, P. aeruginosa, and K. pneumoniae, respectively, we report evidence of functioning efflux pumps using real-time Nile Red assays and enhanced biofilm formation. Further substantiation of the latter is provided through docking and molecular dynamics (MD) simulation studies, which offer insights about the direct interactions of RND efflux pumps with AI-2, the major quorum-sensing molecule of E. coli. Results described here implicate that after transmission, possibly via horizontal gene transfer or e-DNA within bacteria, the assembled efflux pump components could drive multiple aspects of AMR, including its dissemination and ability to adapt to alternate lifestyles such as biofilms, facilitating better survival.
Additional Links: PMID-41603438
Publisher:
PubMed:
Citation:
show bibtex listing
hide bibtex listing
@article {pmid41603438,
year = {2026},
author = {Bhowmik, P and Shanbhag, AP and Venkatesan, S and Bharatham, N and Datta, S and Ramachandran, V},
title = {Modeling and Functional Characterization of Reconstituted Efflux Pump Components from Heterologous Gram-Negative Bacteria.},
journal = {ACS infectious diseases},
volume = {},
number = {},
pages = {},
doi = {10.1021/acsinfecdis.5c00612},
pmid = {41603438},
issn = {2373-8227},
abstract = {Efflux pumps operating in bacteria continuously evolve and contribute significantly toward the rising global trends in antimicrobial resistance (AMR). Our earlier studies demonstrated that the expression of tripartite resistance nodulation division (RND) efflux pump containing the outer membrane protein (OMP), membrane fusion protein (MFP), and inner RND pump from different Gram-negative bacteria results in elevated minimum inhibitory concentrations (MICs) of different antibiotics. Interestingly, parts of this complex could be transferred either within the species or across genera. Despite limited sequence homology, we report the existence of significant structural and functional conservation between the distantly related MFP and RND proteins. Following the assembly of MFP components (AcrA, MexA, OqxA) and RND components (AcrB, MexB, OqxB) from E. coli, P. aeruginosa, and K. pneumoniae, respectively, we report evidence of functioning efflux pumps using real-time Nile Red assays and enhanced biofilm formation. Further substantiation of the latter is provided through docking and molecular dynamics (MD) simulation studies, which offer insights about the direct interactions of RND efflux pumps with AI-2, the major quorum-sensing molecule of E. coli. Results described here implicate that after transmission, possibly via horizontal gene transfer or e-DNA within bacteria, the assembled efflux pump components could drive multiple aspects of AMR, including its dissemination and ability to adapt to alternate lifestyles such as biofilms, facilitating better survival.},
}
▼ ▼ LOAD NEXT 100 CITATIONS
ESP Quick Facts
ESP Origins
In the early 1990's, Robert Robbins was a faculty member at Johns Hopkins, where he directed the informatics core of GDB — the human gene-mapping database of the international human genome project. To share papers with colleagues around the world, he set up a small paper-sharing section on his personal web page. This small project evolved into The Electronic Scholarly Publishing Project.
ESP Support
In 1995, Robbins became the VP/IT of the Fred Hutchinson Cancer Research Center in Seattle, WA. Soon after arriving in Seattle, Robbins secured funding, through the ELSI component of the US Human Genome Project, to create the original ESP.ORG web site, with the formal goal of providing free, world-wide access to the literature of classical genetics.
ESP Rationale
Although the methods of molecular biology can seem almost magical to the uninitiated, the original techniques of classical genetics are readily appreciated by one and all: cross individuals that differ in some inherited trait, collect all of the progeny, score their attributes, and propose mechanisms to explain the patterns of inheritance observed.
ESP Goal
In reading the early works of classical genetics, one is drawn, almost inexorably, into ever more complex models, until molecular explanations begin to seem both necessary and natural. At that point, the tools for understanding genome research are at hand. Assisting readers reach this point was the original goal of The Electronic Scholarly Publishing Project.
ESP Usage
Usage of the site grew rapidly and has remained high. Faculty began to use the site for their assigned readings. Other on-line publishers, ranging from The New York Times to Nature referenced ESP materials in their own publications. Nobel laureates (e.g., Joshua Lederberg) regularly used the site and even wrote to suggest changes and improvements.
ESP Content
When the site began, no journals were making their early content available in digital format. As a result, ESP was obliged to digitize classic literature before it could be made available. For many important papers — such as Mendel's original paper or the first genetic map — ESP had to produce entirely new typeset versions of the works, if they were to be available in a high-quality format.
ESP Help
Early support from the DOE component of the Human Genome Project was critically important for getting the ESP project on a firm foundation. Since that funding ended (nearly 20 years ago), the project has been operated as a purely volunteer effort. Anyone wishing to assist in these efforts should send an email to Robbins.
ESP Plans
With the development of methods for adding typeset side notes to PDF files, the ESP project now plans to add annotated versions of some classical papers to its holdings. We also plan to add new reference and pedagogical material. We have already started providing regularly updated, comprehensive bibliographies to the ESP.ORG site.
ESP Picks from Around the Web (updated 28 JUL 2024 )
Old Science

Weird Science
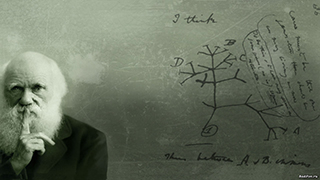
Treating Disease with Fecal Transplantation
Fossils of miniature humans (hobbits) discovered in Indonesia
Paleontology

Dinosaur tail, complete with feathers, found preserved in amber.
Astronomy

Mysterious fast radio burst (FRB) detected in the distant universe.
Big Data & Informatics

Big Data: Buzzword or Big Deal?
Hacking the genome: Identifying anonymized human subjects using publicly available data.